Recombinant antibodies are created using recombinant DNA technology, which involves generating them in vitro using synthetic genes introduced into mammalian cell lines. Not only does this eliminate the need for animal immunization, but re1combinant antibody technology also overcomes the batch-to-batch variability caused by hybridoma cell culture undergoing genetic drift. Another notable advantage of recombinant antibodies is the flexibility to produce different formats (including single chain variable fragment (scFv), Fab fragments and bispecific antibodies), making them amenable to different applications in therapeutics, diagnostics, and fundamental research.
Founded in 2010, evitria delivers recombinant antibody expression services to customers globally. Our vast experience and expertise in recombinant antibody production have resulted in the purification of over 20,000 antibodies. This article details 7 aspects of recombinant antibody technology, including their benefits and applications.
1. What is a recombinant antibody?
Recombinant antibodies consist of heavy and light chains combined by peptide linkers, and use their variable region as receptor to bind to an antigen. The key difference between the generation of recombinant antibodies and traditional antibody production is that the process of recombinant antibody generation does not require animal immunization or the cultivation of hybridomas. As recombinant antibodies are produced from a known DNA sequence, they have enhanced reproducibility compared to polyclonal or traditional hybridoma-based monoclonal antibodies.
Polyclonal antibody preparations report that only a small fraction of antibodies (0.5–5%) generated by immunization of rabbits can recognize the intended target1. The finite and non-renewable nature of polyclonal antibodies limits the amount of specific antibodies that can be isolated. Hybridoma cell culture can also hamper reproducibility as long-term storage can mean hybridoma cell lines can lose their ability to grow — leading to the loss of crucial sources of monoclonal antibodies. In contrast, the synthetic production of recombinant antibodies with defined sequences produced in mammalian cells can produce highly specific and reproducible antibodies on a large scale, with a standardized workflow.
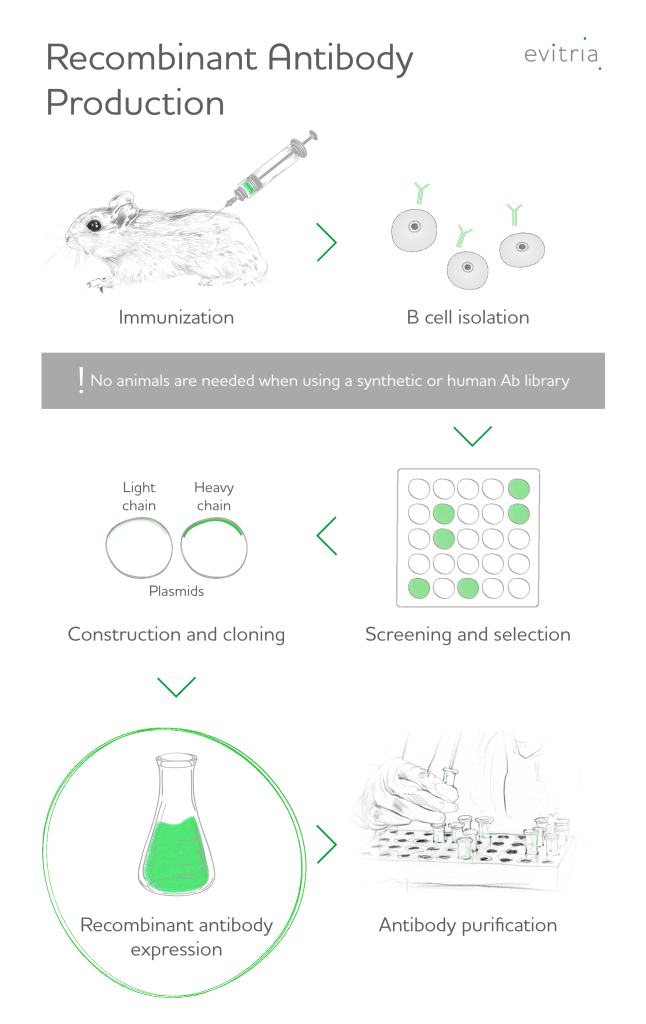
2. Recombinant antibody production
Recombinant antibody production commences with the isolation of promising genetic material (nucleic acids) coding for antibody candidates or using a library of genes with randomised antigen binding site sequences for antibody engineering. Antibody genes are introduced into expression vectors that display the associated antibodies on their surface (antibody phage display library technique).
A bacteriophage library can be employed to screen antibodies to an immobilized antigen. This panning technique eliminates the weak binders and selects for the strong binders, which adhere to the antigen. Repetition of this validation process with increasingly stringent conditions results in the selection of only the most potent and most specific antibodies in the antibody library, with high affinity to a specific epitope. The in vitro process allows the manipulation of the genes to create new antibodies, in order to reduce immunogenicity or even only select antibody fragments (e.g., fab fragments or scFv, i.e. single-chain variable fragment).
Next, the most promising antibody genes are cloned into suitable host cell lines that function as expression platforms. Both prokaryotic and eukaryotic cell types are used as expression systems. As antibodies require post-translational modification, such as the assembly of disulfide bridges, mammalian cell culture are more suitable than prokaryotic cells like Escherichia Coli (E. Coli). Accounting for approximately 70% of all recombinant protein production today, Chinese hamster ovary cells are the predominant mammalian host for therapeutic proteins.
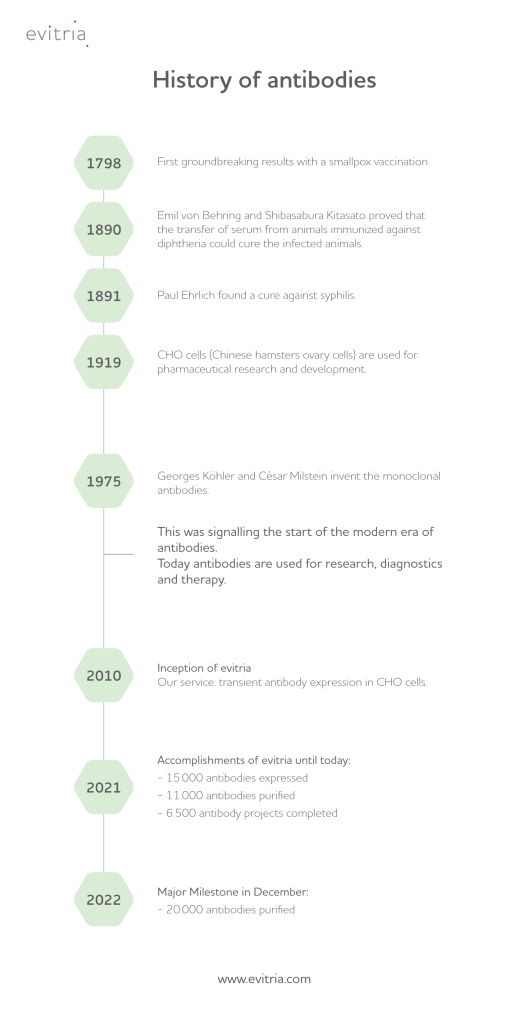
3. Key historical time points of recombinant antibodies
The use of antibodies was first recognized in 1890 by Dr Emil von Behring and Shibasabura Kitasato, who demonstrated that serum from immunized animals could cure others with diphtheria, highlighting the potential of polyclonal antibodies. This discovery led to the development of antibody drugs and vaccines. In 1897, Paul Ehrlich’s “side-chain model” further clarified the specific binding nature of antibodies to antigens.
The Chinese Hamster (Cricetulus griseus) was chosen as a laboratory model for biomedical research in 1919 because of its small size, short gestation period, and low chromosome number2. Today, recombinant antibody technology involves the extensive use of cell lines derived from ovarian cells from this model — Chinese Hamster Ovary (CHO) cells —for obtaining heterologous protein proteins3.
Later in the 1970s, Köhler and Milstein developed the hybridoma method to produce large quantities of specific monoclonal antibodies by fusing antibody-producing B cells with immortal myeloma cells, thus creating unlimited quantities of a specific monoclonal antibody. This was a significant advancement due to their reduced cross-reactivity compared to polyclonal antibodies. Since then, monoclonal antibodies have been developed to target specific mutations and protein defects in several diseases. Advances in recombinant engineering and genetic sequencing have rapidly produced chimeric, humanized, and fully human monoclonal antibodies. Today, the antibody market is rapidly growing, with innovative products like recombinant and bispecific antibodies leading the way.
However, there is much more to learn about the history of antibodies and the several milestones in this field that proved their immense relevance for life sciences and medicine, revolutionizing both areas significantly.
4. Applications of recombinant antibodies
Recombinant antibodies are used in numerous applications in medicine and life sciences:
Approved biologic therapeutics against cancer, autoimmune diseases and infections. Currently, many biologics are recombinant protein therapies, such as monoclonal antibodies. Several bispecific antibodies have been approved by the FDA, with an array of multispecific antibodies currently undergoing clinical trial evaluation.
Diagnostic tools in lateral flow test kits, ELISA, and other immunoassays. For diagnostics and therapy, several recombinant antibodies have been generated against bacteria, viral pathogens, eukaryotic pathogens and toxins4.
Tools for fundamental research (e.g., Western blots, immunofluorescence, flow cytometry, immunohistochemistry) and protein structure analysis. Milligram quantities of recombinant antibodies make crystallography more straightforward and reproducible than antibodies obtained from hybridoma-based expression and purification5.
5. Advantages of recombinant antibodies
Advantages of recombinant antibodies at a glance:
- Scalability
- In vitro & animal-free
- High quality and reproducibility
- Customizable
Find out more about the advantages:
Scalability
Once a recombinant antibody is established and the optimal genetic sequence is known, the antibody can be produced at different scales with high concentrations and more predictable behaviour. Further, the use of well-established expression platforms facilitates the upscaling of the necessary manufacturing processes. Current industrial IgG production levels in the commonly employed CHO cell line often exceed 12 g/L, which has been due to improved high-producer cell lines, optimized production media and reagents as well as prolonged production processes6.
What is an antibody sequencing service?
In vitro process — animal-free
Recombinant antibody production is an entirely in vitro process, making it an agile technology that can switch between individual assignments at short notice. In contrast, animal immunization or production through hybridoma cell lines is more time-consuming. Additionally, in vitro processes are more economical and ethical since they do not require as many resources, produce as much waste, or use animals in the production process. At evitria, expected project timelines for new antibodies using our Recombinant Antibody Expression Service are typically 5 weeks.
Ensures high quality and reproducibility
Since the underlying genetic material is easily optimized through the phage display method, recombinant antibodies can be designed to exhibit superior affinity, sensitivity and specificity over traditional monoclonal antibodies.
Hybridoma cell lines are prone to spontaneous mutations, thus leading to potential consistency issues between batches. Polyclonal antibodies are known for lower reproducibility since each batch is derived from individual animals. In contrast, the production of recombinant antibodies relies on entirely defined and well-controlled genetic sequences and thus yields highly consistent antibody products. This process leads to excellent reproducibility and consistency between batches.
Customizable
Once the amino acid sequence of the antibody is known, it can be edited and customized easily, allowing the properties of the recombinant antibody to be changed for new applications. This is not possible when working with polyclonal or hybridoma-derived antibodies.
6. Customization of antibodies
Recombinant antibodies offer remarkable adaptability because they can be customized and reformatted for specific applications. Evitria specialize in custom recombinant antibody services and offer multiple services for antibody engineering. Below are a few examples of how evitria’s antibody engineering would allow you to produce recombinant antibodies with versatile functions.
As technology evolves, so do recombinant antibodies. Customization and reformatting of recombinant antibodies empower us to tailor their properties for specific applications. Whether it is humanization, chimerization, or creating antibody fragments, recombinant antibodies offer a dynamic canvas for scientific innovation.
Chimerization: Creating Hybrid Antibodies
Chimerization involves combining the variable domains of an antibody (usually from a mouse, rat, or rabbit) with the constant domains from a different species (e.g., human). This results in a chimeric antibody that retains specificity while minimising immunogenicity.
Chimeric antibodies bridge the gap between species, allowing us to harness the best features of each. For example, a humanized chimeric antibody can be used for therapeutic purposes without triggering adverse immune responses.
Humanization: Making Antibodies More Human-Like
Traditional antibodies derived from animals can provoke immune reactions when administered to humans. By grafting the complementarity-determining regions (CDRs) of an antibody onto a human antibody framework, we create humanized recombinant antibodies, which exploit the human portion of the antibody whilst maintaining antigen binding. These retain specificity while minimizing immunogenicity, making them ideal for therapeutic use.
Isotype Switching: Tailoring Antibody Functionality
The isotype of antibodies is determined by the heavy chain constant regions in the immunoglobulins, which are classified as IgG, IgM, IgA, IgD and IgE. The isotype of antibodies is key to their biological function and distribution. Isotype switching allows the alteration of the isotype of an antibody, without affecting the antigen specificity. An example application is converting an IgG to a pentameric IgM isotype for increased avidity. Other reasons to choose isotype switching include overcoming aggregation and altering in vivo effector function, which is advantageous for therapeutic applications as well as applications such as flow cytometry or immunofluorescence to reduce non-specific staining. Evitria’s modular cloning techniques, together with >600 different constant domains, offer isotype switching of your construct to enable fine-tuning of an antibody’s properties.
Antibody Fragments: Compact and Functional
In certain situations, full-length antibodies may not be optimal. Recombinant expression platforms allow us to convert any antibody into fragments, followed by purification. Antibody fragments (Fabs) lack the glycosylated Fc constant regions, provide more rapid tissue penetration, and are less immunogenic. Fab antibodies generated from hybridoma-generated antibodies obtained by IgG proteolysis may not retain the same antigen-binding properties as the whole antibody5, therefore recombinant generation of Fabs is advantageous.
- Single Chain Fv (scFv): Consists of a single variable domain linked by non-covalent forces, useful for research and diagnostics.
- Fab (Fragment antigen-binding): Retains antigen-binding capacity.
- F(ab’)2: Contains two Fab fragments, useful for bispecific antibodies.
Reformatting and engineering your antibody with evitria
If you need a unique format, we are ready to guide you through the design choices.
- Species switching: Beyond human and mouse, we can engineer antibodies for several animals, including human, mouse, rat, rabbit, dog, cat, bovine.
- Formats: Our proprietary cloning system enables reformatting into diverse antibody formats:
- Isotype switching: IgG, IgA, IgE, and IgM and subtype switching
- Reformatting: scFv, Fabs and F(ab’)2, bispecifics
- Fc engineering: Silencing, afucosylation, half-life extension
7. Recombinant antibody importance
The scope of recombinant antibody applications is tremendous. The recent pandemic outbreak of the SARS-CoV-2 virus had a significant impact on everybody’s lives. Strategies to fight the pandemic relied heavily on recombinant antibodies.
One example is the development of therapeutic antibodies that neutralize virus particles in infected patients. Recombinant antibodies that bind to surface proteins of SARS-CoV-2 are used in lateral flow test kits (“antigen tests”) to detect acute infections. A recent publication by Canadian scientists in the Journal of Molecular Biology highlighted the possibility of the synthesis of highly sensitive and specific reporter assays against SARS-CoV-2 virus particles using recombinant antibodies7.
The researchers designed a conjugate reporter system consisting of antibodies coupled to an enzymatic luminescence reporter. The luciferase reporter enzyme’s complementary N- and C-terminal fragments were fused to one of the two antibodies. The simultaneous binding of the two antibodies to target the S-protein resulted in the reconstruction of luciferase activity. The antibodies were developed to have affinity to regions of viral surface proteins using recombinant and phage display technologies. This publication illuminated how recombinant antibodies could be rapidly developed into tools for important applications.
Recombinant antibodies from evitria
At evitria, we use our CHO cell antibody production and expression platform to generate recombinant monoclonal antibodies. We can produce custom recombinant antibodies on a large scale within 5 weeks. Antibody production in CHO cells guarantees the highest quality of recombinant antibodies and fast expression.
Would you like to know more about our services? Please get in touch with us for further information.
Poster Download: antibody development and quality control by mass spectometry
The production of high quality, reproducible material is critical for the development of antibody-based therapeutics. The evitria-Genovis workflow combines rapid, high quality, antibody production with high-throughput mass spectrometry for greater insight and control of key quality attributes.
Download the poster to learn more.

References
- Nilsson, P. et al. Towards a human proteome atlas: High-throughput generation of mono-specific antibodies for tissue profiling. Proteomics 5, (2005). ↩︎
- Yerganian, G. The biology and genetics of the Chinese hamster. in Molecular Cell Genetics 3–36 (Wiley New York, 1985). ↩︎
- Oka, M. S. & Rupp, R. G. Large-scale animal cell culture: a biological perspective. Bioprocess technology vol. 10 Preprint at https://doi.org/10.1201/9780203749166-3 (1990). ↩︎
- Kuhn, P. et al. Recombinant antibodies for diagnostics and therapy against pathogens and toxins generated by phage display. Proteomics – Clinical Applications vol. 10 Preprint at https://doi.org/10.1002/prca.201600002 (2016). ↩︎
- Basu, K., Green, E. M., Cheng, Y. & Craik, C. S. Why recombinant antibodies — benefits and applications. Current Opinion in Biotechnology vol. 60 153–158 Preprint at https://doi.org/10.1016/j.copbio.2019.01.012 (2019). ↩︎
- Frenzel, A., Hust, M. & Schirrmann, T. Expression of recombinant antibodies. Frontiers in Immunology vol. 4 Preprint at https://doi.org/10.3389/fimmu.2013.00217 (2013). ↩︎
- Fellouse, F. A., Miersch, S., Chen, C. & Michnick, S. W. Structure-based Design of a Specific, Homogeneous Luminescence Enzyme Reporter Assay for SARS-CoV-2. J Mol Biol 433, (2021). ↩︎