The production and manufacturing of bispecific antibodies (bsAbs) pose unique challenges and opportunities in the biopharmaceutical industry. With their innovative ability to target two distinct antigens, bispecific antibodies require specialized processes to ensure high yield, quality, and efficacy. This involves careful consideration of bispecific antibody design and engineering strategies to optimize their therapeutic potential.
At evitria, we leverage our expertise in recombinant antibody expression services to advance bispecific and multispecific antibody development, unlocking new therapeutic possibilities. Join us as we explore the intricacies of bispecific antibody production and the innovations shaping this important field.
Bispecific antibody manufacturing: Complexity and purpose
What makes bispecific antibody production so complex is the structure of bsAbs. This sets them apart from traditional monoclonal antibodies and poses distinct challenges to their manufacturing process.
The format of traditional antibodies consists of heavy and light domains connected to form chains, with light chains consisting of 2 immunoglobulin domains and heavy chains consisting of 4 immunoglobulin domains. The common light chains and heavy chains together form a pair in a Y-shaped molecule with two heavy-light chain pairs comprising an antibody.
There are two main functional parts of antibodies (Figure 1): the Fc region, also known as the tail, and the binding sites (Fab region). Responsible for mediating the effector functions, the fragment crystallizable region (Fc) region leads to immune-mediated target cell-killing. The Fab regions contain the variable regions that are the antigen-binding sites. Traditional antibodies are monospecific and bivalent, with the two binding regions targeting the same epitope.
In contrast to conventional therapeutic antibodies, bsAbs recognise two different epitopes on the same or different antigens (e.g. on the cell surface of cancer cells). These bispecific molecules enable the simultaneous targeting of multiple antigens, effector cells, or disease targets, presenting exceptional opportunities in various therapeutic and diagnostic applications, for instance in cancer immunotherapy1.
However, correct heavy and light chain pairing is a substantial challenge in bispecific antibody production, requiring dedicated and innovative approaches.
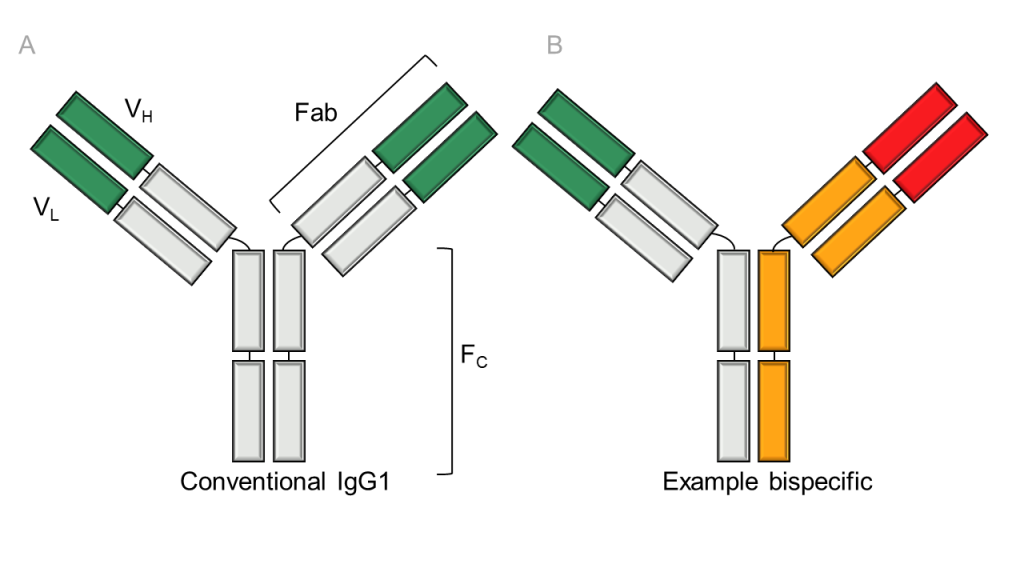
Figure 1: Structure of a bispecific antibody: Bispecific antibodies are able to bind two types of antigen. The format shown is based on immunoglobulin G (IgG) structure (A), which contains a Fab (fragment antigen-binding) region connected to an Fc (fragment crystallisable) region. Two variable domains (VH and VL) provide antigen specificity.; (B) Example of an IgG-based bispecific antibody with Fab regions recognising two antigens. Recreated from2
How are bispecific antibodies made?
The production of bispecific antibodies involves sophisticated in vitro protein engineering and advanced production techniques. Below is an overview of the key aspects of their production process:
1. Antibody engineering and cloning
Bispecific antibodies are engineered by fusing two different monoclonal antibodies (mAbs) or antibody fragments with desired specificities. Using recombinant DNA technology, genes encoding the variable domains of each specific antibody are cloned to create a single construct, resulting in bispecific formats like scFv, diabody, or Fab.
2. Expression systems for antibody production
BsAbs are produced using various expression systems, including mammalian cells, yeast, or bacterial systems. Mammalian cell lines such as CHO cells are commonly used for therapeutic applications due to their ability to perform complex post-translational modifications necessary for antibody functionality.
3. Transfection and protein expression
The bispecific antibody genes are introduced into the expression system through transfection, initiating the production of bispecific antibody molecules. The expression system then synthesizes the bispecific proteins, including both the heavy and light chains.
4. Hybridoma fusion or recombinant expression
For quadroma or bivalent formats, hybrid hybridomas are formed by fusing two different hybridomas that produce the parent mAbs. Alternatively, recombinant techniques like knobs-into-holes mutations facilitate the formation of heavy chain heterodimerization, leading to asymmetric bsAbs.
5. Protein purification
BsAb production involves multiple purification steps to obtain highly pure and active molecules. Techniques such as ion exchange chromatography, protein A affinity chromatography, or peptide tagging are employed to isolate and enrich bsAbs from the expression system.
6. Antibody characterisation and quality control
After antibody purification, bispecific antibodies undergo rigorous characterization and quality control assays. These assays assess their binding specificity, stability, and effector functions. The bispecific antibodies are also evaluated for their pharmacokinetics, half-life, and ability to engage with target cells.
Improving bispecific antibody production
Bispecific antibody production is a complex and intricate process, necessitating cutting-edge protein engineering and purification techniques. The ability to generate bispecific molecules with desired functionalities, including prolonged half-life (e.g. IgG1 format) and asymmetric structures, is a cornerstone in the continuous improvement of these biologics.
Moreover, modulating the half-life of bispecific antibodies is essential to optimize their pharmacokinetics and therapeutic efficacy. Incorporating Fc region modifications or employing half-life extension technologies helps to tailor the antibody’s persistence in vivo, providing sustained therapeutic benefits. This is the case for the CD19 HLE BiTE format —used to treat CD19-positive malignancies — where fusing to an Fc domain significantly increases its serum half-life17.
Ongoing efforts in clinical development are being made to enhance the efficacy and applicability of bsAbs, aiming to maximize their therapeutic potential and reduce potential challenges as well as to optimize immunogenicity. Advanced antibody engineering techniques, such as site-directed mutagenesis and rational design, enable precise control over antibody-antigen interactions.
Aggregation is another common challenge in bispecific antibody development that can compromise the yield, shelf-life and efficacy of a bsAbs. Cell culture conditions, the types of bioreactors and purification methods are optimised to minimise aggregation and ensure consistent product quality.
Design of different types, formats, and structures of bispecific antibodies
Bispecific molecules come in various types, formats, and structures (Figure 2), offering a wide range of tools for targeted therapeutics and diagnostics. The diverse antibody engineering strategies employed to produce bispecific antibodies result in unique functionalities tailored to specific applications.
Due to the existence of the Fc regions, bsAbs can be broadly divided into two categories: IgG-based bsAbs and Fragment-based bsAbs. The first IgG-based bsAbs have a similar structure to conventional antibodies and contain an Fc region, which is associated with multiple activities such as Antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC) and antibody-dependent cell phagocytosis (ADCP).
Inclusion of the Fc region can increase the half-life of the antibody as the receptor can be recognised by the neonatal receptor, which is involved in regulating the IgG serum levels and actively prolonging the biological half-life. The presence of the Fc region can also facilitate their purification and promote their stability and solubility11. The disadvantages of IgG-like bispecific antibodies are the side effects associated with off-target to Fc receptors (FcRs) and chain-associated issues arising from the production or function of these complex molecules due to the presence of multiple antibody chains12. Optimisation of bispecific antibody production involves engineering techniques like knobs-into-holes format to ensure correct heavy chain assembly.
The second primary type of bsAbs is fragment-based bsAbs (also known as single-chain variable fragments scFvs), composed of variable light and heavy domains from two abs, or Fab units, joined by a flexible polypeptide linker but lacking an Fc region. This lack of Fc region has several advantages, including high yield, low cost and good tumor penetration. However, the lower molecular weight means the disadvantages of a faster half-life. Antibody engineering techniques can also improve the half-life and efficacy of fragment-based bsAbs.
Different types of full-size IgG-like bsAbs
- Quadroma formats: Quadroma technology is based on the fusion of two hybridomas to produce bispecific antibodies with heavy and light chains from both parent hybridomas. Although representing the foundation of bispecific antibody production and allowing for enhanced stability and affinity, the quadroma format suffers from low production yields and high heterogeneity13. More specifically, there is only a one-in-ten chance of producing functional bsAbs following the random assembly of two heavy and light chains14.
- CrossMab format: Invented by Roche, CrossMab technology involves exchanging sequences of the heavy and light chain domains of the Fab fragments. This approach ensures correct heavy-light chain association by preventing the formation of unwanted side products.
- Knobs-into-Holes: Developed by Genentech, this format aims to promote Fc heterodimerisation and utilises a large amino acid to create a “knob” structure on one chain and a small amino acid to create a “hole” structure on the other chain. This subsequently enables the correct assembly of two heterologous antibody heavy chains, thus eliminating chain-associated issues12.
Different types of fragment-based bsAbs formats
- Dual affinity re-targeting (DART) format is an example of T-cell recruiting bsAbs. This format lacks the Fc region and is based on the single-chain variable fragment (scFv) structure, consisting of the VH of the first variable region linked to the VL from the second antibody. The second Fragment variable (Fv) consists of the inverse. The stability of the antibody was improved by the addition of a cysteine at the C-terminus of the polypeptide chains to form an intrachain disulphide bond15. Advantages of DART include maintenance of potency in vivo administration due to the structural replication of the natural interaction within an IgG molecule.
- Bispecific T-cell engager (BiTE) format connects endogenous T-cells (via CD3) to tumor-expressing antigens to activate the cytotoxic potential of the patient’s own T-cells. There are two binding domains with 2 scFv regions from a monoclonal antibody linked by a flexible linker. Data from clinical trials prove the efficacy of BiTE in recruiting T-cells and clearing tumors, even at very low doses16.
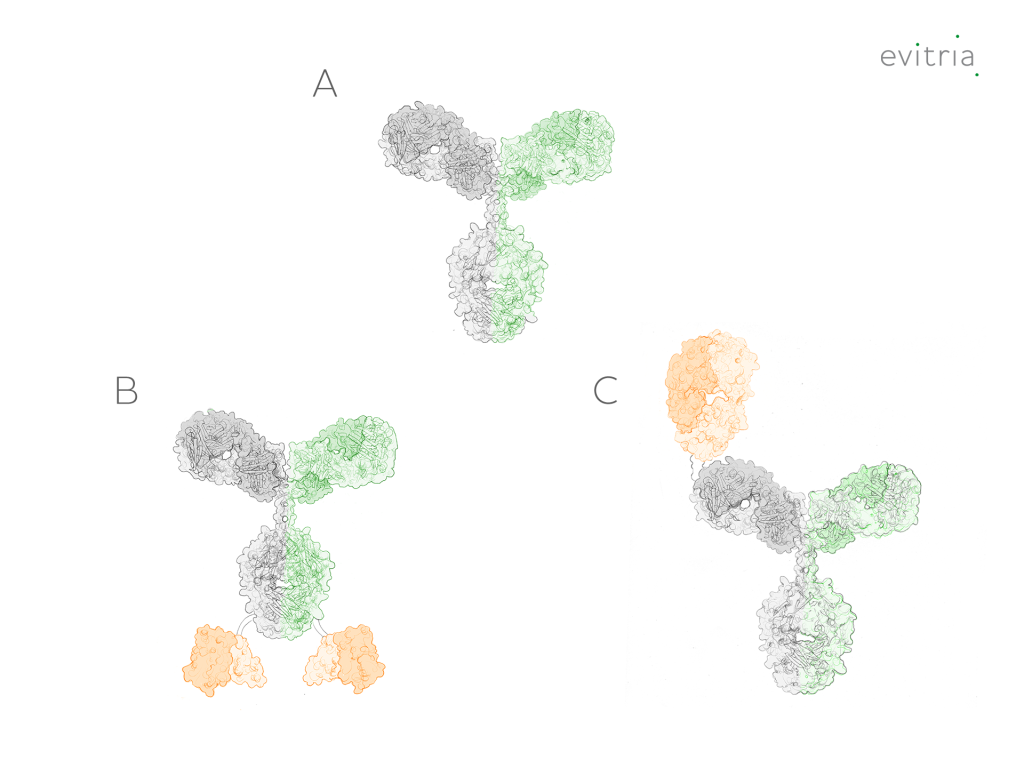
Figure 2: Bispecific antibody formats – (A) traditional format; (B and C) variations with additional binding domains
Bispecific antibody engineering and production at evitria
Considering the complexity of the manufacturing process of bsAbs, several biopharmaceutical companies and research teams outsource the production of bispecific antibodies to CDMOs. Nevertheless, this process requires profound expertise and a large amount of resources.
evitria partners with leading international research and clinical teams to produce bispecific antibodies routinely. In a recent publication in collaboration with the Department of Experimental and Clinical Medicine in Italy and collaborating partners, evitra produced a novel asymmetrical (2 + 1) bispecific T-cell engager (BTCE) targeting CD1a and CD3. The results of the study published in the journal cancers demonstrated that the novel BTCE bsAb was a highly effective therapeutic option for targeting T-cell acute lymphoblastic leukaemia — a rare and aggressive disease — and resulted in the significant inhibition of human T-ALL xenografts.
evitria can provide comprehension experience in transient recombinant antibody production services. With a strong focus on individualisation, we can produce several bispecific antibody types that meet the high standards of our partners and regulatory requirements.
History of the production of bispecific antibodies
The three methods for producing bispecific antibodies have been exemplified in history:– chemical conjugation of two existing abs, fusing hybridomas and producing recombinant proteins.
Bispecific development began in 1961 when Nisonoff et al., employed reoxidation to link the Fab fragment from two different rabbit antibodies3. In 1975, Milstein and Kohler made the crucial scientific breakthrough of fusing B lymphocytes from the spleen to myeloma cells from immunised mice to generate monoclonal antibody-producing myeloma cells.
Milstein went on to produce the first bsAb containing an intact IgG structure by fusing two hybridoma cells in 1983. The bispecific effector functions were reported by Perez in 1985, with the bsAbs shown to bind T-cell receptors and tumor-specific antigens to target tumoral sites and induce T-cell-mediated cytotoxicity for tumors by enhancing antibody-dependent cell-mediated cytotoxicity (ADCC)4. Over the last two decades, there has been a significant shift in the industry towards genetic engineering of proteins, which has revolutionised bsAbs production, resulting in a range of different options regarding origin, composition and production system5.
Read more about bispecifics from Desmond Schofield
- Bispecific Antibodies – Complete Guide
- Bispecific Antibody Formats
- Bispecific Antibody Expression Service
- FDA Approved Bispecific Antibodies
References
1. Ma, J. et al. Bispecific Antibodies: From Research to Clinical Application. Frontiers in Immunology vol. 12 Preprint at https://doi.org/10.3389/fimmu.2021.626616 (2021).
2. Taylor, N. Bispecific Antibody Platforms Spawn Dealmaking Boom Progress in Tackling Technical Challenges in the Development of Bispecific Antibodies and Emerging Opportunities in the Red-Hot Immuno-Oncology Space Have Catalyzed a Flurry of Dealmaking Activity for Bispecific Platform Companies. (2016).
3. Nisonoff, A. & Rivers, M. M. Recombination of a mixture of univalent antibody fragments of different specificity. Archives of Biochemistry and Biophysics vol. 93 Preprint at https://doi.org/10.1016/0003-9861(61)90296-X (1961).
4. Perez, P., Hoffman, R. W., Shaw, S., Bluestone, J. A. & Segal, D. M. Specific targeting of cytotoxic T cells by anti-T3 linked to anti-target cell antibody. Nature 316, (1985).
5. Kontermann, R. E. Dual targeting strategies with bispecific antibodies. mAbs vol. 4 Preprint at https://doi.org/10.4161/mabs.4.2.19000 (2012).
6. Heiss, M. M. et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial. Int J Cancer 127, (2010).
7. Weidle, U. H., Kontermann, R. E. & Brinkmann, U. Tumor-antigen-binding bispecific antibodies for cancer treatment. Semin Oncol 41, (2014).
8. U.S. Food and Drug Adminstration. Bispecific Antibodies: An Area of Research and Clinical Applications. https://www.fda.gov/drugs/spotlight-cder-science/bispecific-antibodies-area-research-and-clinical-applications (2024).
9. Boerman, O. C., Van Schaijk, F. G., Oyen, W. J. G. & Corstens, F. H. M. Pretargeted radioimmunotherapy of cancer: Progress step by step. Journal of Nuclear Medicine vol. 44 Preprint at (2003).
10. Parashar, A., Sarkar, S., Ganguly, A., Sharma, S. K. & Suresh, M. R. Bispecific Antibodies for Diagnostic Applications. in Bispecific Antibodies (2011). doi:10.1007/978-3-642-20910-9_19.
11. Kontermann, R. E. & Brinkmann, U. Bispecific antibodies. Drug Discovery Today vol. 20 838–847 Preprint at https://doi.org/10.1016/j.drudis.2015.02.008 (2015).
12. Sun, Y. et al. Bispecific antibodies in cancer therapy: Target selection and regulatory requirements. Acta Pharmaceutica Sinica B vol. 13 Preprint at https://doi.org/10.1016/j.apsb.2023.05.023 (2023).
13. Liu, H., Saxena, A., Sidhu, S. S. & Wu, D. Fc engineering for developing therapeutic bispecific antibodies and novel scaffolds. Frontiers in Immunology vol. 8 Preprint at https://doi.org/10.3389/fimmu.2017.00038 (2017).
14. Brinkmann, U. & Kontermann, R. E. The making of bispecific antibodies. mAbs vol. 9 Preprint at https://doi.org/10.1080/19420862.2016.1268307 (2017).
15. Johnson, S. et al. Effector cell recruitment with novel Fv-based dual-affinity re-targeting protein leads to potent tumor cytolysis and in vivo B-cell depletion. J Mol Biol 399, (2010).
16. Kang, J., Sun, T. & Zhang, Y. Immunotherapeutic progress and application of bispecific antibody in cancer. Frontiers in Immunology vol. 13 Preprint at https://doi.org/10.3389/fimmu.2022.1020003 (2022).
17. Arvedson, T. L. et al. Abstract 55: Generation of half-life extended anti-CD33 BiTE® antibody constructs compatible with once-weekly dosing. Cancer Res 77, (2017).