As of today, 12 bispecific antibodies (bsAbs) have received FDA approval, marking significant milestones in the advancement of targeted therapies for complex diseases such as cancer and autoimmune disorders. These approved bispecific antibodies are reshaping treatment landscapes by offering more precise and effective solutions.
This article offers insights into the special characteristics of bsAbs, their fields of application, as well as a list of FDA-approved bispecific antibodies and a perspective at current fields of research.
List of FDA approved bispecific antibodies
Here is a detailed list of FDA-approved bsAbs, along with information on their commercial name, manufacturer, and applications:
- Blinatumomab (Blincyto®): Produced by Amgen, blinatumomab targets CD19 and CD3. It is used to treat B-cell precursor acute lymphoblastic leukemia (ALL). Approved in 2014.1
- Emicizumab (Hemlibra®): Produced by Genentech/Roche, emicizumab bridges activated factor IXa and factor X. It is approved for the treatment of hemophilia A. Approved in 2017.3
- Amivantamab (Rybrevant®): This Janssen Biotech antibody targets EGFR and MET. It is indicated for non-small cell lung cancer (NSCLC). Approved in 2021.2
- Tebentafusp (Kimmtrak®): Distributed by Immunocore, tebentafusp targets gp100 and CD3. It is approved for HLA-A*02:01-positive patients with unresectable or metastatic uveal melanoma. Approved in 2022.1
- Faricimab-svoa (Vabysmo®): Produced by Genentech/Roche, Faricimab-svoa targets VEGF-A and Angiopoietin-2. It is used for wet age-related macular degeneration (AMD) and diabetic macular edema (DME). Approved in 2022.4
- Teclistamab (Tecvayli®): Produced by Janssen Biotech, Teclistamab targets BCMA and CD3. It is used for relapsed or refractory multiple myeloma. Approved in 2022.2
- Mosunetuzumab (Lunsumio®): Developed by Genentech/Roche, Mosunetuzumab targets BCMA and CD3. It is approved for relapsed or refractory follicular lymphoma. Approved in 2022.3
- Epcoritamab (Epkinly®): A collaboration between Genmab and AbbVie, Epcoritamab targets CD20 and CD3. It is approved for treating diffuse large B-cell lymphoma (DLBCL) and – more recently – follicular lymphoma. Approved in 2023.4
- Glofitamab (Columvi®): Also produced by Genentech/Roche, Glofitamab targets CD20 and CD3. It is indicated for relapsed or refractory diffuse large B-cell lymphoma, or large B-cell lymphoma arising from follicular lymphoma. Approved in 2023.5
- Talquetamab (Talvey®): Produced by Janssen Biotech, Teclistamab targets GPRC5D and CD3. It is used for relapsed or refractory multiple myeloma. Approved in 2023.6
- Elranatamab (Elrexfio®): Made by Pfizer, elranatamab targets BCMA and CD3 for treatment of multiple myeloma. Approved in 2023.7
- Tarlatamab (Imdelltra®): Produced by Amgen, tarlatamab targets DLL3 and CD3 for the treatment of extensive stage small cell lung cancer. Approved in May 2024.8
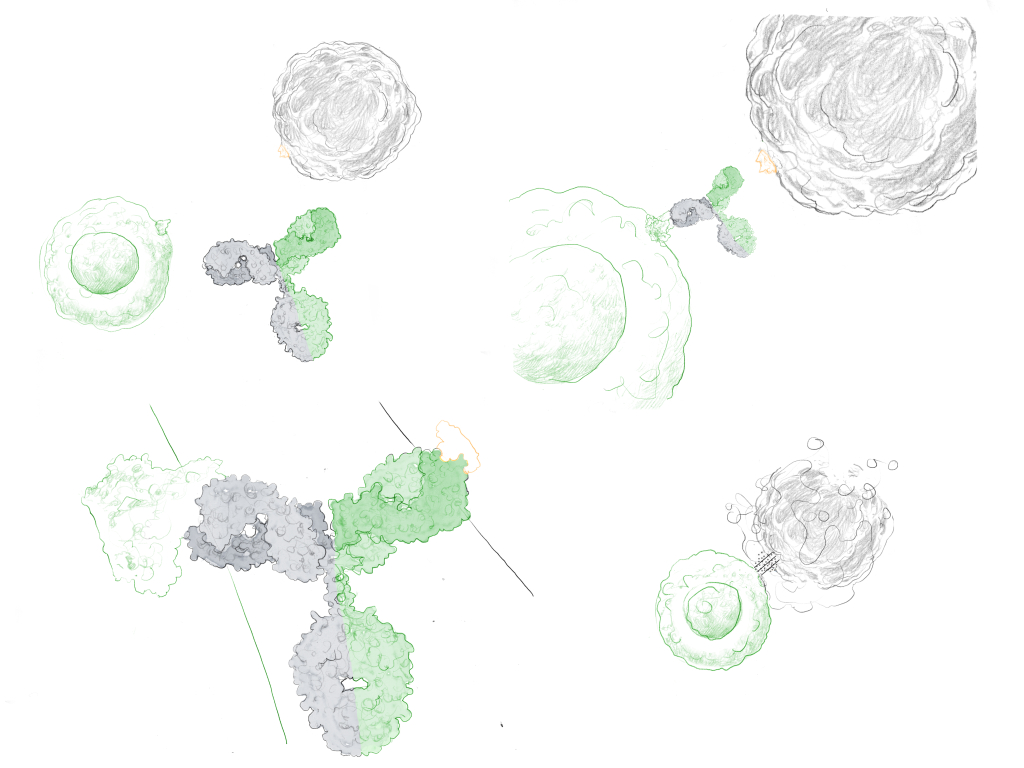
Bispecific antibodies – what makes them stand out?
BsAbs are unique due to their ability to bind two different antigens simultaneously. This structure allows them to perform functions that monoclonal antibodies are not able to, such as bringing two different cell types into close proximity to facilitate an immune response. For instance, a bsAb can guide a T-cell to a cancer cell, enabling the T-cell to attack the cancer cell more directly.
Thus, the advantages of bispecific antibodies are substantial. BsAbs can target multiple pathways in a disease process, making them extremely versatile and effective. They can also reduce the number of medications a patient needs to take, simplifying treatment regimens.
Additionally, by engaging multiple targets, bsAbs can help overcome resistance mechanisms that might limit the effectiveness of single-target therapies. This makes them particularly valuable in treating complex diseases such as cancer.
Current and future fields of application for bsAbs
BsAbs are proving revolutionary across multiple fields of medicine due to their ability to target two different antigens simultaneously. This is one of the main reasons for the multiple fields of application of bispecific antibodies.
In oncology, bsAbs play a crucial role by targeting cancer cells on multiple pathways or by simultaneously recruiting immune cells to attack tumors, thereby enhancing the effectiveness of cancer treatments. Examples of bispecific antibodies include blinatumomab (Blincyto®) and amivantamab (Rybrevant®), which are used to treat various types of cancer.910, In hematology, bsAbs are used against conditions like hemophilia A by connecting clotting factors and thereby improving coagulation, as in the case of emicizumab (Hemlibra®).11
Additionally, in the field of ophthalmology, bsAbs like faricimab (Vabysmo®) are used to treat ocular conditions such as wet age-related macular degeneration (AMD) and diabetic macular edema (DME), offering new hope for patients with these vision-threatening diseases.12
At the moment, intense research is being carried out to develop bsAbs not only for cancer, but also neurodegenerative, ocular, and vascular conditions. Further fields of research include chronic inflammatory, hematologic, and autoimmune diseases.13

State-of-the-art bsAb production – by evitria
evitria is at the forefront of bispecific antibody production, utilizing state-of-the-art techniques to deliver high-quality antibodies for both research and therapeutic applications. Specializing in the transient expression of antibodies in CHO cells, evitria offers a range of services tailored to meet the unique needs of their clients – from research groups to pharma companies and CDMOs. evitria combines advanced production methods with extensive expertise to ensure efficient and reliable antibody production.
Our approach leverages transient transfection techniques in CHO cells, enabling rapid production cycles with high yields and minimal endotoxin levels. This is particularly important for generating complex proteins with human-like post-translational modifications, which are essential for the functionality and efficacy of therapeutic antibodies.
evitria’s custom bispecific antibody production service also includes support in deciding on the most suitable bispecific antibody type, and is designed to accommodate various formats and technologies, such as Knobs-into-Hole and CrossMab, ensuring that each project meets your specific requirements.
Read more about Bispecific Antibodies from Desmond Schofield
- Bispecific antibody production – A comprehensive overview
- Bispecific Antibody Expression Service
- Bispecific antibodies simply explained
- Understanding the side effects of bispecific antibodies
- Development of bispecific antibodies
- How to choose a bispecific antibody format?
- Bispecific antibody formats
- What companies are developing bispecifc antibodies?
Sources
- Kimmtrak®. Prescribing information. Immunocore Limited; 2022. Accessed July 9, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761228s000lbl.pdf ↩︎
- Tecvayli®. Prescribing information. Janssen Biotech, Inc; 2023. Accessed July 9, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761291s000lbl.pdf ↩︎
- Lunsumio®. Prescribing information. Genentech Inc; 2022. Accessed July 9, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761263s000lbl.pdf ↩︎
- Epkinly®. Prescribing information. Genmab US, Inc and AbbVie, Inc; 2023. Accessed July 9, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761324s000lbl.pdf ↩︎
- Columvi®. Prescribing information. Genentech Inc; 2023. Accessed July 9, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761309s000lbl.pdf ↩︎
- Talvey®. Prescribing information. Janssen Biotech, Inc; 2023. Accessed July 9, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761342s000lbl.pdf ↩︎
- Elrexfio®. Prescribing information. Pfizer Inc; 2023. Accessed July 9, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761345Orig1s000lbl.pdf ↩︎
- Imdellra®. Prescribing information. Amgen Inc; 2024. Accessed July 9, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761344s000lbl.pdf ↩︎
- Blincyto®. Prescribing information. Amgen Inc; 2018. Accessed June 14, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125557s013lbl.pdf ↩︎
- Rybrevant®. Prescribing information. Janssen Biotech, Inc; 2021. Accessed July 9, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761210s000lbl.pdf ↩︎
- Hemlibra®. Prescribing information. Genentech Inc; 2024. Accessed June 27, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761083s018lbl.pdf ↩︎
- Vabysmo®. Prescribing information. Genentech Inc; 2022. Accessed June 14, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761235s000lbl.pdf ↩︎
- U.S. Food And Drug Administration. Bispecific Antibodies: an area of research and clinical applications. Published February 14, 2024. Accessed June 14, 2024. https://www.fda.gov/drugs/spotlight-cder-science/bispecific-antibodies-area-research-and-clinical-applications. ↩︎