Therapeutics development
We provide you with highly specific antibodies, supporting you with the development of novel therapeutics.


Recombinant antibodies for drug development
Our custom antibody expression service offers high specific, top-notch products exclusively designed to meet your unique needs, empowering you to pioneer novel solutions and drive forward scientific breakthroughs.
We do not only manufacture antibodies – with more than 13 years of experience, our experts can also offer valuable guidance for your project, providing a deeper insight into the mechanisms of action of our different antibody products.
Our capabilities in developing therapeutic antibodies at your disposal
Since 2010, we have preformed more than 120,000 antibody production runs, expressed more than 23,000 proteins, and purified more than 20,000 antibodies. Access our expertise in the following areas:
- Fusion antibodies including Fc fusion proteins
- Isotype switching
- Chimerization
- Glyco-engineering
- Bispecific and trispecific antibodies
We make your challenges our mission
Lead identification
Time
Budget
Scalability
“Quality and reproducibility considerations are often delayed until later in vitro and early in vivo experiments. However, this may lead to irreproducible results, and miss out on identifying the best lead
candidates.”

Dr. Desmond Schofield
Director of Business Development at evitria
Our expression service by clinical stage
The production of high quality, reproducible material is critical for the development of antibody-based therapeutics. From antibody discovery to lead selection and in vivo studies, our dedicated services in antibody generation provide you with recombinant antibodies at any stage, tailored to your specific needs in terms of quality and speed of delivery.
DISCOVER
Basic research
Discovery

Candidate screening
Accelerated screening support
Reproducible quality for future scale-up
Production of 20+ constructs at a scale <5mg
Transient expression in CHO cells
High purity of >95%, low levels of endotoxin (<1 EU/mg)
Preclinical

Lead selection
High consistency of the reproduced candidates
Due diligence & lead optimization: Reformatting, Fc-engineering, subtype switching, isotype switching
Production of approx. 20 constructs at a scale <1,000-mg
Transient expression in CHO cells
High purity of >95%, low levels of endotoxin (<1 EU/mg)
Preclinical

In vivo studies
Record-setting completion times within 4 weeks
High consistency of the reproduced candidates
Production of approx. 3 constructs at a mg to gram scale
Transient expression in CHO cells
High purity of >95%, low levels of endotoxin (<1 EU/mg)
What our customers say
Long-lasting, healthy customer relationships are very important to us. Anonymity is therefore part of our corporate DNA.
“We are happy paying for the assured quality that evitria brings.”
Sr Scientist, Biotherapeutics, Series B Startup, Boston
“evitria is our first choice for high quality antibody production.”
Director, Biotherapeutics, Startup, New York
“We have been working with evitria for several years and highly appreciate the high consistency of the delivered material.”
Sr. Scientist, Biotherapeutics, Switzerland
Proofed quality
At evitria, the quality of the antibodies produced is our top priority. 99% of our projects are successfully completed within the agreed time frame, with the highest purity and zero contamination issues. We support you with highly consistent material, which is of great importance in your pre-clinical stages to find the lead candidate for in vivo studies.
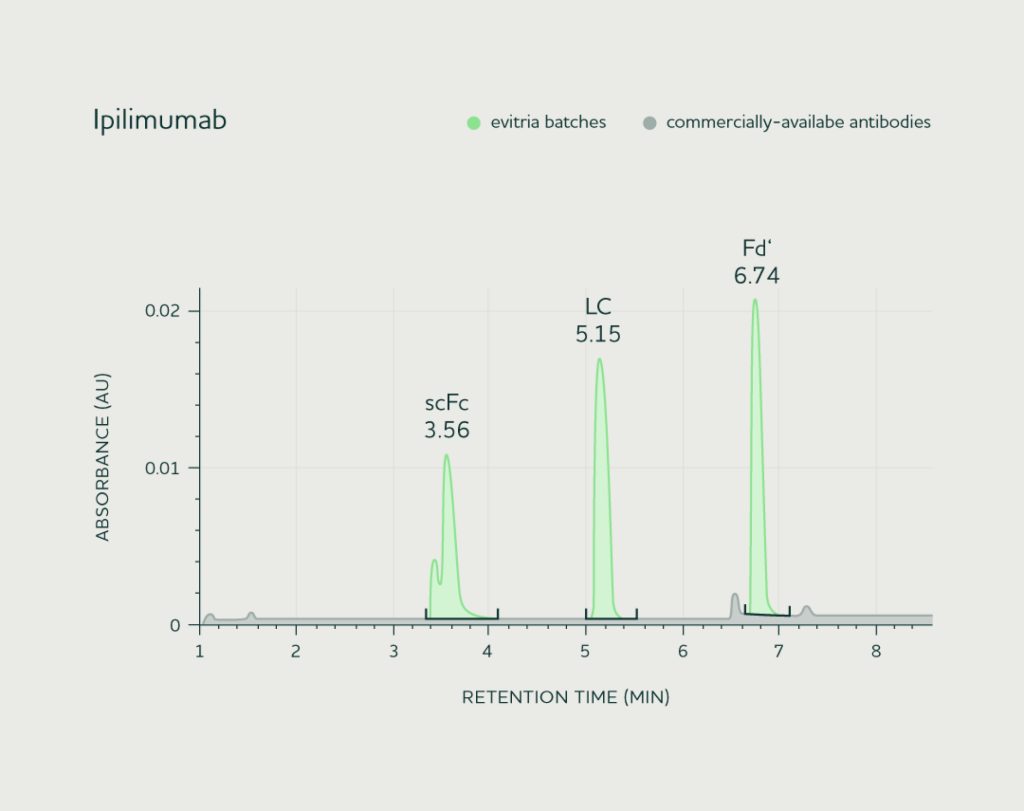
We demonstrated the consistency of material between evitria batches by comparing LC-MS data across three different productions of four commercial antibodies.
Publications with evitria
Our recombinant antibodies have been referenced as material for scientific papers over 40 times from 2022 to 2023 alone.
Find more examples of therapeutic antibody applications using glyco-engineering or bispecific antibody production methods in our section dedicated to the publications with evitria.
Melo, Vinicio, et al. “EGFR-selective activation of CD27 co-stimulatory signaling by a bispecific
antibody enhances anti-tumor activity of T cells.” Front Immunol. Jul 20. 14 (2023):1191866.
https://doi.org/10.3389/fimmu.2023.1191866
Caracciolo, Daniele, et al. “Therapeutic afucosylated monoclonal antibody and bispecific T-cell
engagers for T-cell acute lymphoblastic leukemia.” Journal for immunotherapy of cancer 9.2
(2021). https://doi.org/10.1136%2Fjitc-2020-002026
Get in touch with our experts now!
Our team of experts is looking forward to talk with you about your plans, concerns and expectations regarding our expression services.



