Scalability & Reproducibility
“Start with the end in mind” is the mantra for new product development activities; if you do not have a clear end-goal for your development, the temptation to chase bad results or over-invest in interesting (but non-essential) data can be too much to resist!
Getting it right first time, then again and repeat
At evitria, we always advocate for careful forward planning and the design of production plans to fit your needs. Whether this be in the design of a bispecific panel for an initial feasibility, or the production of large quantities of antibodies for in vivo studies. However, all this planning is irrelevant if you do not have consistency in your material across time and scale. This is what evitria was founded to provide to researchers.
Scalability and Reproducibility with CHO cells
More than 90% of the time, antibody-related formats will be produced in CHO cells for clinical and commercial material supply. This is due to the many benefits of CHO cells, shown below.
At evitria, we pioneered the development of transient CHO services, to enable our clients to “start with the end in mind”, and work with their desired host system from day 1. However, this was not enough – in order to be a reliable partner for biotherapeutic R&D, we have to provide consistent material quality across time and scale.
We can demonstrate this when looking at our production history. Below is an example of a series of productions undertaken for one client. First, a small-scale production to support proof of concept work was delivered in summer 2020.
Then a >2 year development gap followed while initial data and funding was gathered. After this, the client returned for several larger productions to support their later development and in vivo studies.
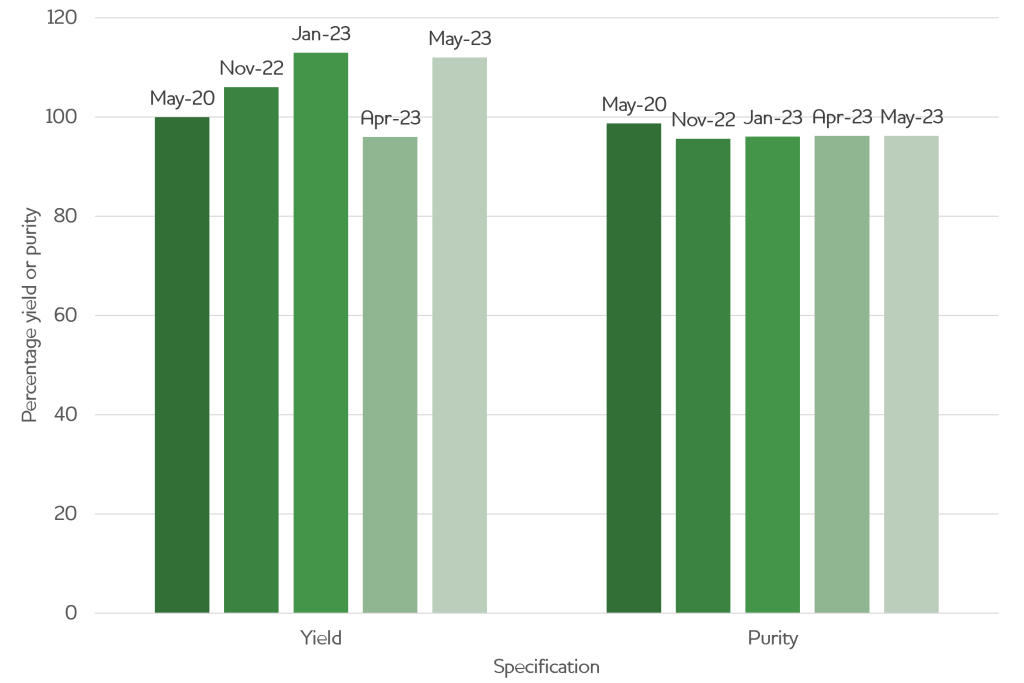
Consistent quality at every scale
Delivering material with the same quality at every scale, and with several years in between campaigns, enabled our client to rapidly move through development stages without having to undertake difficult troubleshooting activities or worry about their material quality.
| Production date | Yield (% of 1st production) | Purity (%) | Scale (L transfected) |
| May-20 | 100 | 98.7 | 0.5 |
| Nov-22 | 106 | 95.6 | 1 |
| Jan-23 | 113 | 96.1 | 5 |
| Apr-23 | 96 | 96.2 | 2 |
| May-23 | 112 | 96.2 | 4 |
Get in touch with our experts now!
Our team of experts is looking forward to talk with you about your plans, concerns and expectations regarding our expression services.



