Over the last three decades, advancements in recombinant antibody engineering have led to the development of monoclonal antibodies (mAbs) with significant clinical benefits, which are employed to treat a myriad of conditions. One notable type of antibody engineering is afucosylation, which involves the removal or absence of fucose residues from the (fragment crystallizable region) Fc region. Afucosylation enhances the binding of antibodies to Fc receptors on immune effector cells and boosts antibody-dependent cellular cytotoxicity (ADCC) for more effective tumour cell killing. In this blog, we will review afucosylated antibodies, discuss their benefits and provide details of our afucosylated antibody expression service.
What are afucosylated antibodies?
Human antibodies are categorized into five primary isotypes: immunoglobulin (Ig)G, IgA, IgM, IgE, and IgD. Both IgG and IgA can be subdivided into subclasses; for IgG, these include IgG1, IgG2, IgG3, IgG4. Although the overall structural framework of antibody molecules is similar across all isotypes, antibody isotypes differ in their ability to bind to Fc receptors on immune cells and activate immune responses.
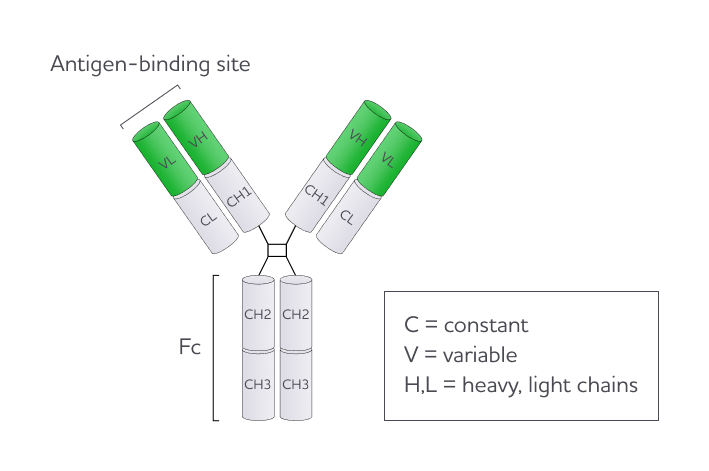
Most therapeutic mAbs are IgG1, not only because of their greater stability but also because the human IgG1 Fc moiety interacts efficiently with the activating Fc gamma receptors (FcɣRs) expressed on the surface of immune cells1 2 3. This leads to Fc-mediated effector functions, including antibody-dependent phagocytosis (ADCC) by natural killer (NK) cells and other immune cells, which can induce target cell death. Cell death occurs due to the release of perforin and granzymes, resulting in the apoptosis of malignant cells.
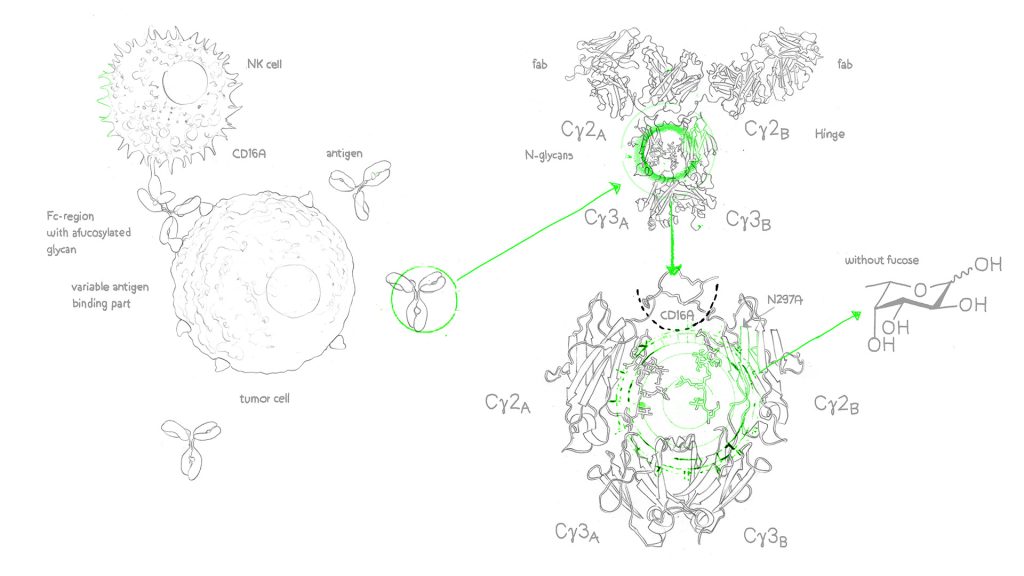
A key site within the Fc region is needed for the Fc domain of the antibodies to interact with FcɣRs on immune cells. This is the N-glycosylation site at asparagine 297 (Asn 297), located in the CH2 domain of each heavy chain. The N-glycans at Asn 297 are mostly complex-type glycans (comprising multiple monosaccharides) and exhibit structural diversity, a phenomenon referred to as microheterogeneity. Despite this variation, a consistent feature present in 90% of complex type IgG N-glycans is the presence of an α-1,6 fucose residue, also known as core fucose4.
In the last two decades, it has been established that removing the IgGs Fc core fucose from mAbs (afucosylated monoclonal antibodies) has proved beneficial for therapeutic IgG1 mAbs and confers enhanced ADCC5 6 7. Afucosylation achieves this by increasing the affinity between the antibody and the FcR receptor in NK cells, which leads to enhanced activation of the signalling molecules, promoting degranulation and increasing cytotoxicity of the NK cells. Overall, this results in an increase in the efficiency of ADCC8. Indeed, the study by Sheilds et al., reported a 50-fold improvement in binding to human FcγRIIIA in fucose-deficient IgG17.
Applications of afucosylated antibodies
Afucosylated antibodies find diverse applications across the field of biomedicine:
- Cancer immunotherapy: Obinutuzumab is an anti-CD20 mAb approved for use with bendamustine hydrochloride for follicular lymphoma in patients who have relapsed or have not responded to rituximab. Although rituximab has improved outcomes for patients with CD20+ malignancies since its approval by the FDA in 1997, responses can vary between patients, and rituximab resistance can develop9 10. Obinutuzumab has a reduced fucose content compared to conventional mAbs like rituximab. This results in increased affinity to FcγRIII-expressing effector cells and a greater capacity to induce NK cell-mediated ADCC (reviewed in 11).
- Allergies: Researchers are exploring afucosylated antibodies’ ability to modulate immune responses. In 2017, the FDA approved benralizumab for severe eosinophilic asthma – a severe type of asthma12. Benralizumab works by binding to the IL-5Rα receptor on eosinophils through its Fab regions. This binding prevents the recruitment of additional eosinophils to the airways of asthmatic patients, which can worsen asthma symptoms due to the cytokine IL-5 interacting with its receptor. The lack of fucose in the CH2 domain of the Fc region allows it to bind at a 5-50 times higher affinity to the FcγRIIIa receptor of NK cells (reviewed in 13). Compared with the original fucosylated antibody, there is an amplification of ADCC, which can induce eosinophil apoptosis by NK cells through the release of perforin and granzyme B (reviewed in 14).
- Infectious Diseases: Binding and blocking the entry of a viral particle into a cell is an essential application of neutralising antibodies. Afucosylated antibodies serve as effective neutralising antibodies. One example is the fucose-free version of the 13F6 monoclonal antibody that serves as an immunoprotectant for Ebola virus infection, displaying superior potency15.
Strategies for afucosylation
Since the first approval of afucosylated & recombinant antibodies for clinical use, artificially cultivated antibodies have seen a steady rise in utilization. There are multiple strategies for reducing the degree of afucosylation or removing all fucose residues, which involves employing genetically altered cell lines, chemical inhibition and expression of recombinant enzymes that disrupt fucose synthesis.
Altering fucosyltransferase FUT8 expression
The enzyme responsible for catalysing the transfer of a fucose residue from GDP-fucose to the innermost N-acetylglucosamine residue on N-glycans for core fucosylation is fucosyltransferase 8 FUT8 (Figure 2). Many strategies that produce afucosylated antibodies involve inactivating the FUT8 gene or downregulating its expression. A FUT8-/-cell line was generated by targeted disruption of FUT8 in the CHO DG44 cell line. This cell line has exhibited a similar growth kinetic and productivity compared to the parental cell lines in 1L bioreactors.16 Differences are the production of completely afucosylated antibodies, which have increased ADCC activity16. Targeting FUT8 mRNA in the CHO DG44 cell line by small interfering RNA (siRNA) has also been effective, producing 60% afucosylation in antibodies with an over 100-fold increase in ADCC activity. Additionally, selected clones remained stable17.
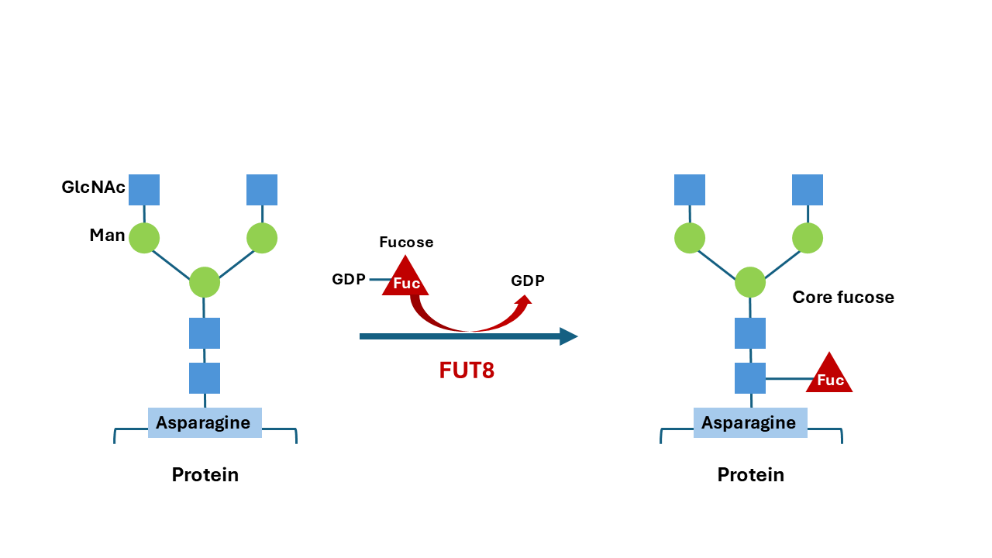
Source: evitria, recreated from 18
Chemical inhibitors
An alternative to genetically engineering cell lines is chemical inhibition. 2-fluorofucose and 5-alkynylfucose derivatives have been employed to produce afucosylated antibodies with enhanced ADCC activities. These small chemical inhibitors are thought to deplete cells of the substrate, GDP-fucose, which is needed by fucosyltransferase 8 (FUT8) to incorporate fucose glycans into the antibody structure19.
evitria’s afucosylated antibody expression service using GlymaxX® technology
Through our exclusive licensing partnership, evitria provide afucosylation services to our customers using ProBioGen’s GlymaxX® technology. Production of afucosylated antibodies via ProBioGen’s GlymaxX® technology could not be more straightforward. This process involves the heterologous expression of the bacterial oxidoreductase GDP-6-deoxy-D-lyxo-4-hexulose reductase (RMD), which redirects an intermediary in the fucose synthesis pathway towards a non-functional dead-end product (Figure 3). Further blocking via the de novo pathway (synthesis of fucose from simple precursors within a cell) may occur by competitive inhibition of GMD by GDP-rhamnose20. evitria supports all stages of the strategy, including determining the degree of afucosylation by measuring the G0/G0F ratio through mass spectrometry.
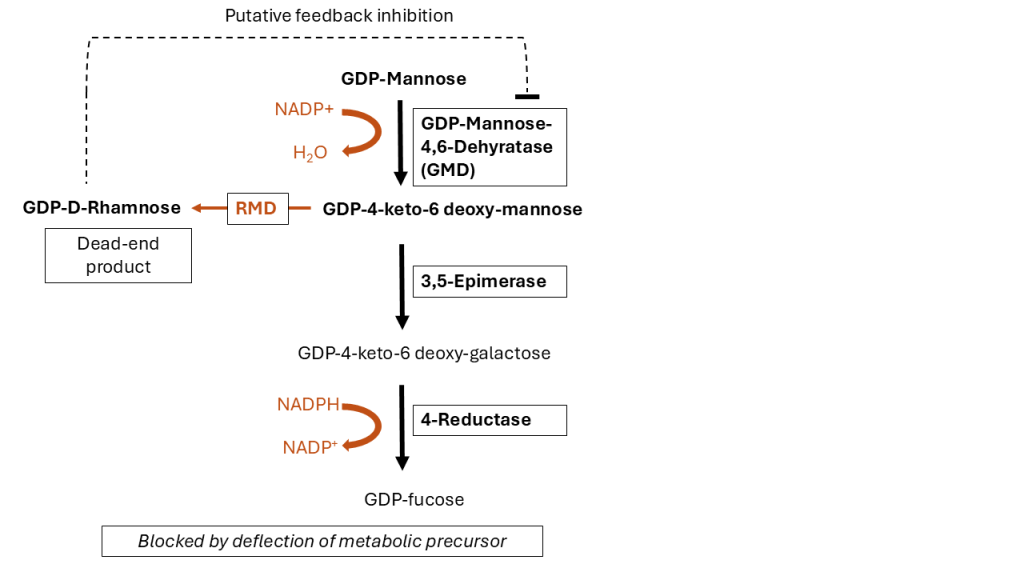
Source: evitria, recreated from 20
Contact evitria for more information on producing afucosylated recombinant antibodies
At evitria, recombinant antibody production is based on the clients’ individual needs and requirements. Evitria specializes in the transient expression of both native and afucosylated antibody variants within Chinese hamster ovary (CHO) cells, leveraging the GlymaxX® technology. evitria has now purified more than 175 GlymaxX® afucosylated antibodies. The yields and stability of afucosylated antibodies match native variants, making them ideal as negative controls. In contrast to strategies relying on Fc mutations, Evitria’s GlymaxX® approach mitigates the risk of immunogenicity.
Example of evitria’s production of afucosyated antibodies
Collaborating with the University of Oslo and Oslo University Hospital in Norway, evitria generated a CD37 targeting humanized IgG1 mAb for potentially treating non-Hodgkin lymphoma (NHL). CD37 is a promising target due to its high expression in the majority of B-cell NHL tissues21. The article by Generalov et al., details the preclinical assessment of a fully afucosylated anti-CD37 antibody (NNV024). Afucosylation enhanced affinity to FcγRs, which directly impacted ADCC activity: NNV024 demonstrated a more than 3-fold enhancement in ADCC activity compared to obinutuzumab in patient-derived chronic lymphocytic leukaemia cells. Furthermore, in vivo data in a B-NHL mouse model revealed a significant improvement in survival compared to obinutuzumab treatment22.
Recommended readings from Desmond Schofield:
Afucosylation sipmly explained
Antibody-Dependent Cellular Cytotoxicity (ADCC) – definition, mechanism & application
Afucosylated Antibody Expression Service
Glycoengineering: pushing forward biotechnology
Frequently Asked Questions
What are afucosylated antibodies?
+What is FcγRIII?
+What is the role of fucosyltransferase?
+- Bruhns, P. et al. Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood 113, (2009). ↩︎
- Pereira, N. A., Chan, K. F., Lin, P. C. & Song, Z. The “less-is-more” in therapeutic antibodies: Afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. mAbs vol. 10 Preprint at https://doi.org/10.1080/19420862.2018.1466767 (2018). ↩︎
- Ito, T. & Tsumoto, K. Effects of subclass change on the structural stability of chimeric, humanized, and human antibodies under thermal stress. Protein Science 22, (2013). ↩︎
- Golay, J., Andrea, A. E. & Cattaneo, I. Role of Fc Core Fucosylation in the Effector Function of IgG1 Antibodies. Frontiers in Immunology vol. 13 Preprint at https://doi.org/10.3389/fimmu.2022.929895 (2022). ↩︎
- Umaña, P., Jean-Mairet, J., Moudry, R., Amstutz, H. & Bailey, J. E. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol 17, (1999). ↩︎
- Shinkawa, T. et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. Journal of Biological Chemistry 278, (2003). ↩︎
- Shields, R. L. et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. Journal of Biological Chemistry 277, (2002). ↩︎
- Liu, S. D. et al. Afucosylated Antibodies increase activation of Fcg RIIIa-dependent signaling components to intensify processes promoting ADCC. Cancer Immunol Res 3, 173–183 (2015). ↩︎
- Freeman, C. L. & Sehn, L. H. A tale of two antibodies: obinutuzumab versus rituximab. British Journal of Haematology vol. 182 Preprint at https://doi.org/10.1111/bjh.15232 (2018). ↩︎
- Grillo-Lopez, A. et al. Rituximab The First Monoclonal Antibody Approved for the Treatment of Lymphoma. Curr Pharm Biotechnol 1, (2005). ↩︎
- Illidge, T. et al. Obinutuzumab in hematologic malignancies: Lessons learned to date. Cancer Treatment Reviews vol. 41 Preprint at https://doi.org/10.1016/j.ctrv.2015.07.003 (2015). ↩︎
- FDA. Benralizumab. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/761070Orig1s000SumR.pdf (2017). ↩︎
- Kolbeck, R. et al. MEDI-563, a humanized anti-IL-5 receptor α mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. Journal of Allergy and Clinical Immunology 125, (2010). ↩︎
- Pelaia, C. et al. Benralizumab: From the basic mechanism of action to the potential use in the biological therapy of severe eosinophilic asthma. BioMed Research International vol. 2018 Preprint at https://doi.org/10.1155/2018/4839230 (2018). ↩︎
- Zeitlin, L. et al. Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc Natl Acad Sci U S A 108, (2011). ↩︎
- Yamane-Ohnuki, N. et al. Establishment of FUT8 knockout Chinese hamster ovary cells: An ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng 87, (2004). ↩︎
- Mori, K. et al. Engineering Chinese hamster ovary cells to maximize effector function of produced antibodies using FUT8 siRNA. Biotechnol Bioeng 88, (2004). ↩︎
- Biointron. Biointron Announces the Official Launch of Afucosylated Antibody Expression Platform! (2023). ↩︎
- Okeley, N. M. et al. Development of orally active inhibitors of protein and cellular fucosylation. Proc Natl Acad Sci U S A 110, (2013). ↩︎
- Von Horsten, H. H. et al. Production of non-fucosylated antibodies by co-expression of heterologous GDP-6-deoxy-d-lyxo-4-hexulose reductase. Glycobiology 20, (2010). ↩︎
- Zhao, X. et al. CD37 Is a Potential Therapeutic Target for B-Cell Non-Hodgkin Lymphoma. Blood 116, (2010). ↩︎
- Generalov, R. et al. NNV024, a novel humanized anti-CD37 antibody with enhanced ADCC and prolonged plasma half-life in human FcRn transgenic mice for treatment of NHL. Preprint at https://doi.org/10.1101/2023.07.12.548667 (2023). ↩︎