Bispecific antibody formats offer innovative solutions by enabling the simultaneous targeting of two distinct antigens, opening new possibilities in therapeutic applications. With various formats available, from tandem scFvs to full-length IgG-based designs, each brings unique advantages and challenges. Having a deep understanding for these formats is crucial for selecting the right approach to meet specific therapeutic goals.
In this article, we’ll explore the different types and formats of bsAbs, their unique features, and therapeutic applications. Additionally, we’ll highlight evitria’s role in advancing bsAb development.
Bispecific antibody structure
There have been more than 100 bispecific antibody formats created, thanks to the modular structure of antibodies. These formats differ in several aspects, such as molecular weight, the number of antigen-binding sites, the spatial arrangement of these sites, valency for each antigen, capacity to support secondary immune functions, and pharmacokinetic half-life. With ongoing advancements in antibody engineering, many more formats are expected to emerge in the future.1
BsAbs can be broadly categorized into those with Fc regions and those without.1 The Fc region is the tail region of an antibody which interacts with and activates the immune system. Fc-containing bsAbs benefit from additional immune functions and longer half-lives, while Fc-lacking bsAbs offer superior tissue penetration and reduced immunogenicity.2
Structural classification of bsAbs
Structurally, bispecific antibodies are classified based on their binding domains, typically categorized by valency and target division. Possible valencies include “1+1” (two binding sites, one for each target), “2+2” (four binding sites, two for each target), and “2+1” (three binding sites, two for one target, one for the other). These structural variations are essential in tailoring bsAbs for specific therapeutic applications.
Types of bispecific antibodies
- Traditional structures resemble conventional monoclonal antibodies but with different binding domains on each arm.
- Enhanced formats include additional binding regions like single-chain variable fragments (scFvs) or fragment antigen-binding (Fab) domains, creating multi-specific molecules.
- Non-antibody bispecifics are formed by linking together antibody fragments without Fc regions, such as individual scFvs, Fab domains, or VHH domains (the variable fragment of heavy chain-only antibodies).
Additional modifications, such as suppressing immune effector functions or integrating other mutations, can enhance the functionality and effectiveness of bsAbs. Selecting the suitable bispecific format requires careful consideration of application requirements, manufacturing procedures, and commercial viability.
At evitria, we facilitate your research journey and assist you in choosing the optimal bispecific format. We specialize in all common bispecific formats and provide high-quality material within a rapid turnaround time of 4 weeks, from sequence to antibody delivery.
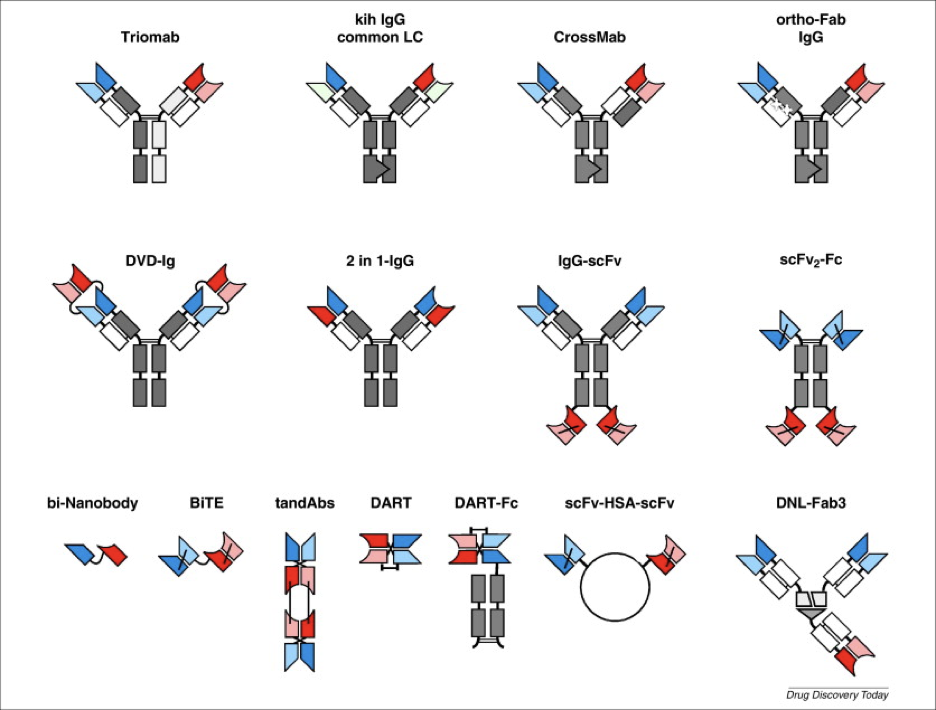
Image: Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Discovery Today. 2015;20(7):838-847. doi:10.1016/j.drudis.2015.02.008. [Erratum, Drug Discovery Today. 2019;1422.]
Bispecific antibody formats with Fc regions
BsAbs with Fc regions combine the structural features of conventional antibodies with the ability to bind two different antigens. This category of bsAbs retains Fc-mediated effector functions, which include antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and antibody-dependent cellular phagocytosis (ADCP). The presence of the Fc region also enhances purification, solubility, and stability3, and often results in longer serum half-lives due to FcRn-mediated recycling.4
Overview of bispecific antibodies with Fc regions:
- Quadromas
- Knobs-into-Holes (KIH)
- Dual-Variable Domains Ig (DVD-Ig)
- IgG-scFv
- Two-in-One or Dual Action Fab (DAF) Antibodies
Quadromas
The quadroma technology involves the fusion of two distinct hybridomas to produce bsAbs. This method can generate bsAbs that closely resemble conventional antibodies.
Key Features:
- The first approved bsAb, Catumaxomab (Removab®), was produced using rat/mouse quadroma cell lines.5
- Despite producing nonfunctional antibodies as by-products, this technology remains a cornerstone in bsAb production.
Knobs-into-Holes (KIH)
This approach addresses the issue of inappropriate homodimer formation by engineering the CH3 domain to create complementary “knob” and “hole” structures in the Fc regions, promoting heterodimerization.
Key Features:
- The KIH technique reduces the risk of unwanted homodimerization.
- Combining this method with other strategies, such as CrossMab technology6, can further enhance specificity and pairing accuracy.
Dual-Variable Domains Ig (DVD-Ig)
Dual-variable domains Ig (DVD-Ig) are constructed by fusing the antigen-binding variable domains of a monoclonal antibody to those of another monoclonal antibody via flexible linkers.
Key Features:
- The Fab arms of a DVD-Ig are symmetrical and can each bind to two different targets, avoiding issues with heavy and light chain mispairing.
- This design improves product homogeneity, yield, and stability, although there may be a risk of reduced binding affinity for the inner variable domain.1
IgG-scFv
IgG-scFv molecules are generated by attaching scFvs or variable single domains to the termini of IgG heavy or light chains.
Key Features:
- This format combines the stability of IgG with the specificity and versatility of scFv.
Two-in-One or Dual Action Fab (DAF) Antibodies
DAF antibodies possess antigen-binding sites which are each capable of recognizing two different antigens.
Key Features:
- Achieving high dual affinity requires significant engineering of the antigen-binding site.
- These antibodies typically can only bind one antigen on each arm, limiting some applications. However, variants like DutaMab and DutaFab can bind both antigens on each arm (if allowed by antigen size and orientation).1
Bispecific antibody formats without Fc regions
BsAbs without Fc regions are smaller in size compared to their IgG-like counterparts. This smaller size can lead to enhanced tissue penetration, making them particularly useful in certain therapeutic applications where deep tissue access is crucial. These formats often consist of smaller antibody fragments or engineered structures that lack the Fc region, resulting in different pharmacokinetic properties and potentially lower immunogenicity.2
Overview of bispecific antibody formats without Fc regions:
- scFv-based bsAbs
- Tandem scFvs
- Diabody Format
- Single-Chain Diabodies
- Tandem Diabodies (TandAbs)
- Dual-Affinity Retargeting Molecules (DARTs)
- Nanobodies
scFv-based bsAbs
scFvs contain the basic elements for antigen binding, comprised of only the variable regions of a heavy and light chain (VH and VL, respectively), joined by a short flexible peptide linker. ScFvs can oligomerize depending on the linker length and antibody sequence.
Key Features:
- scFvs typically have high tumor specificity and tissue penetration.
- Various clinical applications are possible due to their small size.
Tandem scFvs
In this format, two different scFvs are connected in tandem by a flexible peptide linker.
Key Features:
- A short linker prevents intra-chain pairing, enhancing antigen-binding efficiency.
- A long flexible linker allows free rotation of antigen-binding sites.
- This structure forms the basis of the bispecific T cell engager (BiTE) technology.7
Diabody Format
In the diabody format, the variable domains of two different antibodies are connected by two linkers, forming a stable structure. In one polypeptide chain, the VH of the first antibody is linked to the VL of the second antibody. In another chain, the VL of the first antibody is linked to the VH of the second antibody. The VH and VL of each parent antibody form noncovalent bonds to assemble the diabody.
Key Features:
- Two linkers increase stability but can restrict the mobility of antigen-binding sites, limiting antigen recognition.8
Single-Chain Diabodies
Single-chain diabodies are an extension of the diabody format in which the polypeptide chains are joined by an additional peptide linker for increased stability and versatility.
Key Features
- Enhanced stability while retaining small size for better tissue penetration.
Tandem Diabodies (TandAbs)
TandAbs are tetravalent molecules formed by two polypeptide chains which each contain two VL and two VH domains.
Key Features:
- TandAbs have high target avidity and specificity due to bearing multiple binding sites per antigen.
- TandAbs are suitable for applications requiring strong binding to multiple targets.
Dual-Affinity Retargeting Molecules (DARTs)
DARTs are structurally similar to diabodies, with the addition of cysteine at the C-terminus of both chains to promote the formation of a disulfide bond to enhance stability.
Key Features:
- The small size of DARTs increases tissue penetration.
- An Fc fragment can be fused to DARTs (DART-Fc) to prolong their serum retention time, enhancing therapeutic efficacy.9
Nanobodies
Nanobodies are the smallest naturally occurring (in camelids) antigen-binding fragment, consisting only of the variable domain of a heavy chain-only antibody (a VHH domain).
Key Features:
- High stability and solubility due to their small size (~15 kDa).10
- Can be linked together to create bispecific molecules with different binding specificities.

How to choose a bispecific antibody format?
Choosing the right bispecific antibody (BsAb) format is critical for optimizing therapeutic efficacy, safety, and manufacturability. Factors such as the therapeutic objective, target antigen characteristics, pharmacokinetics, immunogenicity, and manufacturing feasibility all play a role in selecting the most suitable format. Each BsAb format comes with unique advantages and challenges, making careful evaluation essential for clinical success. At evitria, we offer expert guidance and cutting-edge production capabilities to help you navigate these complexities and bring your BsAb projects to life.
Read more: How to choose a bispecific antibody format?
Bisepcific antibody development and production with evitria
At evitria we support your bispecific research and development needs. With over 20,000 antibodies and antibody-related molecules purified, we offer expert guidance in selecting and optimizing bispecific formats.
Our capabilities include producing various bsAb structures, from traditional formats to innovative multi-specific designs, ensuring high-quality outcomes for your therapeutic development projects. Trust evitria to help you navigate the complexities of bispecific antibody research and accelerate your path to groundbreaking discoveries.
Read more about Bispecific Antibodies from Desmond Schofield
- Bispecific antibody production – A comprehensive overviewBispecific Antibody Expression Service
- Bispecific antibodies simply explained
- Understanding the side effects of bispecific antibodies
- Development of bispecific antibodies
- How to choose a bispecific antibody format?
- What companies are developing bispecifc antibodies?
- FDA approved bispecifc antibodies
- 1.Brinkmann U, Kontermann RE. The making of bispecific antibodies. mAbs. Published online January 10, 2017:182-212. doi:10.1080/19420862.2016.1268307
- 2.Kang J, Sun T, Zhang Y. Immunotherapeutic progress and application of bispecific antibody in cancer. Front Immunol. Published online October 20, 2022. doi:10.3389/fimmu.2022.1020003
- 3.Carter PJ. Introduction to current and future protein therapeutics: A protein engineering perspective. Experimental Cell Research. Published online May 2011:1261-1269. doi:10.1016/j.yexcr.2011.02.013
- 4.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. Published online August 17, 2007:715-725. doi:10.1038/nri2155
- 5.Linke R, Klein A, Seimetz D. Catumaxomab. mAbs. Published online March 2010:129-136. doi:10.4161/mabs.2.2.11221
- 6.Roche. Biotechnology: CrossMAb technology. Roche. Published 2024. Accessed June 25, 2024. https://www.roche.com/stories/crossmab-technology-in-research-technologies
- 7.van de Donk NWCJ, Zweegman S. T-cell-engaging bispecific antibodies in cancer. The Lancet. Published online July 2023:142-158. doi:10.1016/s0140-6736(23)00521-4
- 8.Kwon NY, Kim Y, Lee JO. Structural diversity and flexibility of diabodies. Methods. Published online February 2019:136-142. doi:10.1016/j.ymeth.2018.09.005
- 9.Root A, Cao W, Li B, et al. Development of PF-06671008, a Highly Potent Anti-P-cadherin/Anti-CD3 Bispecific DART Molecule with Extended Half-Life for the Treatment of Cancer. Antibodies. Published online March 4, 2016:6. doi:10.3390/antib5010006
- 10.Harmsen MM, De Haard HJ. Properties, production, and applications of camelid single-domain antibody fragments. Appl Microbiol Biotechnol. Published online August 18, 2007:13-22. doi:10.1007/s00253-007-1142-2