The production of recombinant antibodies (recombinant antibody expression) involves the use of genetic engineering to produce specific antibodies in vitro. Gene fragments encoding parts of an antibody can be combined and modified to create hybrid antibodies with unique properties.
Recombinant manufacture enables the production of high-quality, homogeneous antibodies with specific properties and functions that can be tailored to different applications. Recombinant antibodies can be designed to target specific epitopes and engineered to improve their stability, specificity, and affinity. The fast production and high quality of recombinant monoclonal antibodies has made them a critical tool in biotechnology and biotherapeutics.
In this article, we will discuss the process of recombinant antibody production, and its advantages and limitations compared to traditional antibody production methods. evitria has specialized in recombinant antibody production services since 2010 and has gained immense experience in the field with more than 120,000 production runs, supporting customers all over the world.
Recombinant antibody production in detail
Antibodies (also known as immunoglobulins [Ig], such as IgG) are proteins that are typically produced by B cells in response to foreign substances, such as viruses or bacteria.
Natural antibodies are composed of two heavy chains and two light chains, and each chain has a constant region and a variable region. These chains arrange into a “Y“ shape. The arms of the “Y“ comprise the parts of the antibody responsible for binding its target, or antigen; these are known as fragment antigen-binding (Fab) regions.1
Read more on antibody production in B cells
Antibody-encoding genes can be expressed in bacterial or mammalian cell expression systems by cloning them into an expression vector, which is introduced to the host cells via transfection. The host cells then produce the antibody, which can be purified and used for various applications, such as diagnostic assays or therapeutic treatments.
Compared to traditional methods of antibody production, recombinant antibody production offers several advantages. For instance, recombinant antibody technology enables the production of large quantities of homogeneous antibodies that can be easily optimized for specific applications. Furthermore, it allows for the generation of humanized or fully human antibodies, which are less likely to trigger an immune response in patients and therefore more suitable for therapeutic use.
Read more: Antibody production services – a comparison
Applications of recombinant technology in therapeutics and diagnostics
Therapeutics
Almost half of all therapeutic proteins are monoclonal antibodies, which have been developed for several clinical indications, including cancers, autoimmunity and genetic disorders2. The complexity of producing biologics is exemplified by the fact that a typical protein drug may require in excess of 5,000 critical process steps, which is a great deal more steps than needed for a small-molecule drug2. However, the consistency of recombinant antibody technology eliminates batch-to-batch variation, aligning with the strict manufacturing standards required for therapeutic antibodies.
Diagnostics
Recombinant antibodies also play an essential role in diagnostics, as monoclonal antibodies are employed in workflows that diagnose pathologies as well as routine laboratory investigations, such as immunochemistry and flow cytometry technologies3. These workflows require the high specificity of antibodies to differentiate specific antigenic epitopes for prompt assessment. Recombinant technology is well-suited to the demands of diagnostic assay design — where lower molecular weight versions of antibodies may be advantageous over full-length fragments due to their enhanced thermal stability and solubility4.
Formats of recombinant antibodies
Human IgG antibody
Another advantage of recombinant antibodies is that they can be ‘engineered’. Antibody engineering is the technique of producing antibodies with the desired specifications but in varying formats. The most commonly marketed format is the full-length IgG molecules, whose structure is depicted in Figure 1.
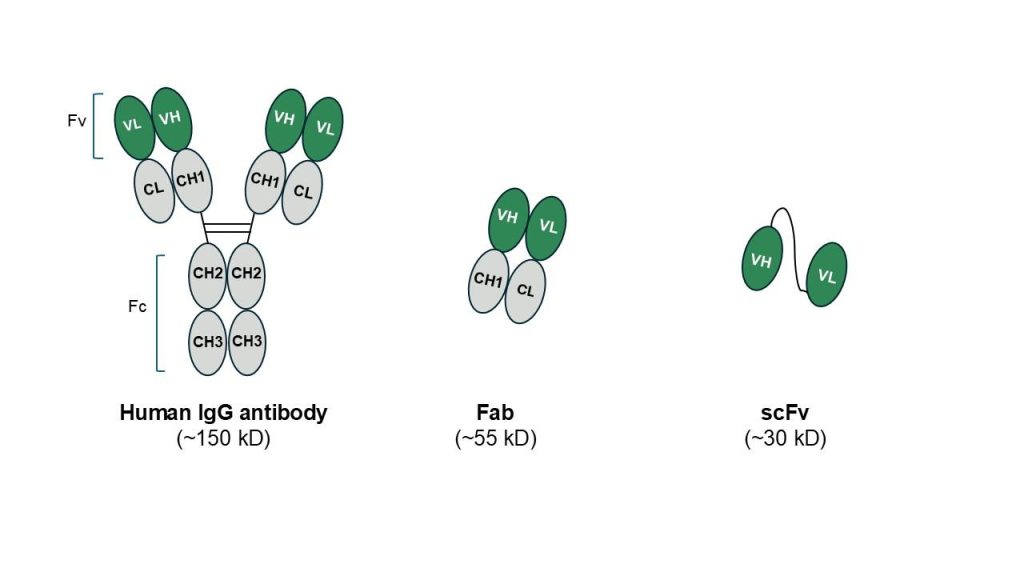
Figure 1. Popular recombinant antibody formats. Reproduced from 5
The structure of an IgG antibody consists of a pair of identical heavy and light chains linked by disulphide bonds. Heavy chains contain three constant domains (CH1, CH2 and CH3), whilst light chains contain only one constant domain. The antigen-binding site comprises variable domains from both the heavy and light chains (VH and VL, respectively). The effector functions of antibodies implicate the Fc region (tail region consisting of paired CH2 and CH3 domains), which interacts with various immune components. This can lead to antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC), which, when employed for therapeutic applications, can enable effective induction of tumour cytotoxicity. Rituximab is an example of a therapeutic antibody that triggers both ADCC and CDC responses to enable effective cell lysis in follicular and aggressive B-cell non-Hodgkin lymphomas (reviewed in 5).
The Fc region is also essential for the protein’s half-life, which protects the antibody from degradation via binding of the Fc region with neonatal Fc receptor (FcRn). Indeed, fusion of the Fc region to biologically active proteins (e.g. cytokines, enzymes) with short half-lives has proven helpful in extending a drug’s circulating half-life. This is particularly important for avoiding frequent dosing regimes that could negatively impact well-being, particularly in vulnerable patient types2.
Antibody fragment engineering
The presence of Fc can lead to deleterious consequences in specific clinical applications. For that reason, smaller recombinant antibody fragments that lack the Fc region may have superior properties for diagnostics and therapeutics. Modifying the antibody sequence and structure to make smaller fragments that lack the Fc region can enhance the specificity, affinity, stability, and compatibility with the human immune system.
Fv and single-chain Fv (scFv) antibody fragments
Fv and single-chain Fv (scFv) antibody fragments (Figure 1) represent classes of antibodies with smaller molecular weights. These antibody fragments retain the targeting specificity of full-length mAbs but cannot initiate effector functions due to the lack of the Fc region.
Fv antibody fragments consist of the antibodies’ VH and VL domains, which are connected by non-covalent interactions. These are less stable than the scFv format, engineered by joining the VH and VL domains via a flexible peptide linker (10-20 aa). Many linker designs are available, with the most popular being the highly flexible (Gly4Ser)3. Further engineering advancements involve additional cysteine residues at the interface to create disulphide-stabilised Fv (dsFv) antibody fragments.
Producing scFv antibody fragments is more cost-effective than full-length antibodies. In addition, scFv antibody fragments exhibit properties ideal for specific diagnostic applications6. For example, the intravenous administration of scFv exhibits accelerated biodistribution and fast clearance compared to full-length versions, making them ideal for rapid nuclear medicine imaging applications involving short-lived isotopes (Tc-99m)7. Other advantages include better penetration in IHC and Enzyme-linked Immunosorbent Assay (ELISA) applications.
Other formats
Fab fragment antibodies: The Fragment antigen-binding (Fab) format is a popular antibody design consisting of the complete light chain along with the variable region and one constant domain of the heavy chain. Notable FDA-approved therapeutics employing this design include abciximab, ranibizumab and certolizumab pegol.
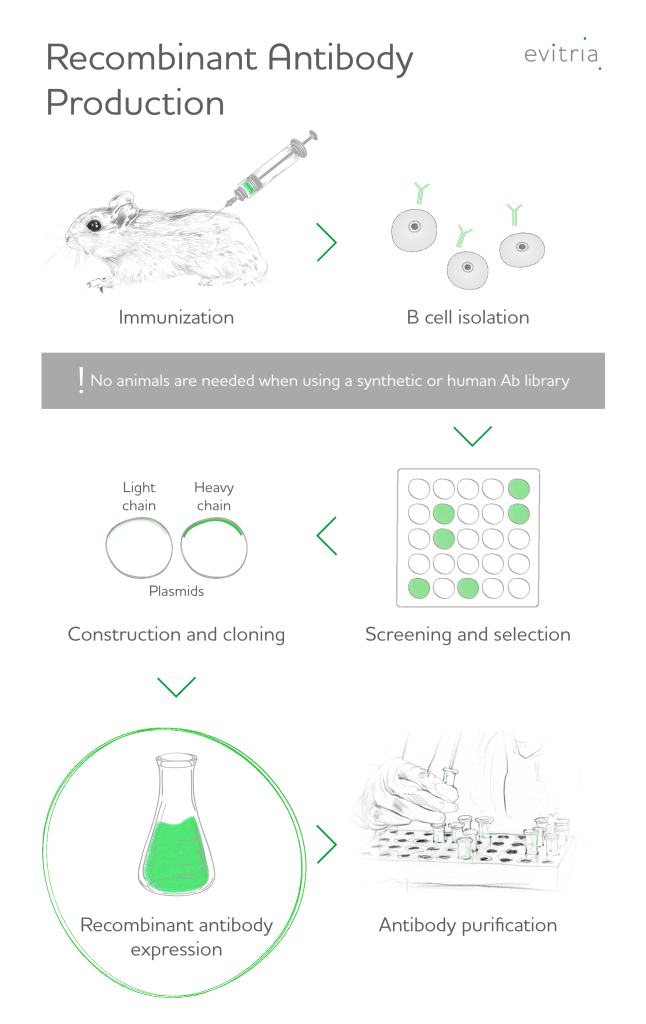
Steps in recombinant antibody production
The steps involved in recombinant antibody production typically include:
- Identification of target antigen: considerations are target accessibility and expression levels. Examples of targets include receptors on tumour cells.
- Generation of antibody library: This library contains a collection of different Fab fragments, each with a unique sequence that can potentially recognise the target antigen. The library can be generated in various ways, such as by isolating antibodies from B-cells in an immunised donor organism.
- Screening for antibodies: The antibody library is screened to identify the fragments that bind specifically to the target antigen. This can be done using techniques such as phage display or yeast surface display.
- Antibody engineering and optimization: Once the antibodies have been identified, they can be engineered and optimised to improve their stability, specificity, and affinity for the target antigen. This can be done using techniques such as site-directed mutagenesis or antibody fusion.
- Expression and purification of antibodies: The final steps involve expression and purification. Recombinant antibody expression can be undertaken using bacterial or mammalian cell expression systems. Purification of the recombinant antibodies is achieved using techniques such as chromatography-based purification.
Antibody engineering and optimization
Antibody engineering and optimization is a critical step in recombinant antibody production, as it enables the generation of antibodies with improved properties for various applications. This step typically involves the use of genetic engineering techniques to modify the sequence and structure of the antibody molecule.
There are several strategies that can be used for antibody engineering and optimization, including:
Antibody humanization
Antibodies produced in non-human species can elicit an immune response when administered to humans. Antibody humanization involves the modification of the antibody sequence to better mimic antibodies found naturally in humans. This reduces immunogenicity and increase compatibility with the human immune system.8
Affinity maturation
Affinity maturation involves the optimization of the antibody’s binding affinity for its target antigen. This can be achieved by introducing mutations into the antibody sequence that enhance its interaction with the antigen.
Bispecific antibody production
Bispecific antibodies are designed to simultaneously bind to two different antigens. This can be achieved through the fusion of antibody fragments containing different Fab regions, or the use of bispecific scaffolds.9
Read more on bispecific antibody production
Antibody fragment engineering
Antibody fragments are smaller antibody molecules that retain the antigen-binding properties of the full-length antibody. Antibody fragment engineering involves the modification of the fragment sequence to improve its stability, specificity, and affinity.
Antibody fusion
Antibody fusion involves the fusion of an antibody molecule with another protein or peptide to create a molecule with new properties. For example, antibody fusion can be used to create immunoconjugates that combine the targeting properties of the antibody with the therapeutic properties of a drug.10
Overall, antibody engineering and optimization can greatly improve the utility, efficacy and safety of recombinant antibodies for various applications. By modifying the antibody sequence and structure, it is possible to create molecules with enhanced specificity, affinity, stability, and compatibility with the human immune system.
Host production systems for recombinant antibodies
Antibody-encoding genes can be expressed in various systems, including bacteria, yeast, and mammalian cells. The choice of expression system depends on several factors, such as the desired antibody format, expression yield, and post-translational modifications required for functionality. The plasmids used for antibody production can also impact the expression and purification process.
Bacterial expression systems
Bacterial expression systems have advantages such as fast growth, ease of genetic manipulation, and simple and inexpensive growth conditions. Gram-positive bacteria, such as Bacillus subtilis — which lack an outer membrane and possess a cell wall — effectively secrete recombinant proteins into the medium, which is required for downstream processing.
Gram-negative bacteria, such as Escherichia coli (E. coli), are favourable as they can express a broad range of heterologous proteins, making them effective for industrial-scale production11. The shortcoming of bacterial systems is the difficulty of producing full-length glycosylated antibody structures due to the lack of organelle structures responsible for post-translational modification.
Another issue with bacterial protein expression is that, in some cases, recombinantly produced protein can aggregate into inclusion bodies. The consequent refolding of proteins from inclusion bodies can be time-consuming, can require protocol optimisation, and prote12ins need to be properly characterised to verify the native protein structure is maintained13.
Yeast expression systems
Yeast is a single-cell eukaryotic organism with the cellular machinery required to perform complex post-translational modifications required for recombinant antibody production, such as glycosylation and disulphide bond formation. Pichia pastoris is easy to handle, cheap, and amenable to high-density fermentation. The drawback of recombinant protein expression in yeast is the differences in glycosylation. In S. cerevisiae, expressed proteins have N- and O- hyperglycosylation, which may cause immunogenicity14.
Mammalian expression systems
Mammalian expression systems remain the most popular option, with 70% of recombinant therapeutic proteins produced in Chinese hamster ovary (CHO) cells15 12. CHO cells have several advantages over other systems: glycosylation is similar to human cells, and the cells are not susceptible to human virus infection. Other benefits are that the cells can be grown to high density, and serum-free conditions can be employed, decreasing the risk of contamination and being favourable for downstream protein purification processes16. Recombinant antibodies produced in mammalian systems have high biological activity and stability. The drawbacks of mammalian expression systems are that they are more expensive and time-consuming than bacterial or yeast systems.
Transient and stable expression
Recombinant antibodies can be expressed in cells either transiently or stably. Transient antibody expression involves introducing genetic material into cells for a short period and harvesting the expressed protein after a few days. This method is faster and easier but results in lower expression yields. Stable expression involves integrating the antibody gene into the host cell genome, resulting in higher expression yields but a longer lead time and a more complex production process.
Post-expression processing
After expression, recombinant antibodies may require additional processing steps, such as purification, characterisation and validation. Purification removes undesired components and contaminants and can be achieved using various methods, such as chromatography, ultrafiltration and precipitation.
At evitria, we provide comprehensive downstream processing services, including affinity chromatography based on protein A and other resins, specialised columns, and protein polishing methods like gel filtration and ion exchange chromatography. Protein characterisation is an essential step in verifying the antibody’s structure, specificity, and functionality, typically using techniques such as ELISA, Western blotting, flow cytometry and mass spectrometry.
Read more: Antibody production services at evitria
Advantages and limitations of recombinant antibody production
Recombinant antibody production offers several advantages over traditional methods of monoclonal antibody production, such as hybridoma technology, particularly in terms of scalability, reproducibility, and versatility.
Advantages of recombinant antibody production
- Production of large quantities: Recombinant antibody production allows for large-scale production of antibodies that are homogeneous and consistent in quality. Further, they can be useful in high-throughput screening and other applications.
- Easy engineering and optimization: Recombinant antibodies can be easily engineered and optimized to improve their binding affinity, specificity, and stability.
- Humanization: Recombinant antibody production enables the generation of humanized or fully human antibodies, which have reduced immunogenicity and are better suited for therapeutic use.
- Versatility: Recombinant antibodies can be engineered to have various formats and properties.
Limitations to recombinant antibody production
- Complexity: Recombinant antibody production is a complex process that requires specialised equipment and expertise.
- Cost: The cost of recombinant antibody production can be high due to the need for specialised equipment and expertise.
- Production time: Recombinant antibody production can be time-consuming, with production times ranging from several weeks to several months, depending on the service provider. At evitria, we manage projects in 5 weeks, from sequence to antibody production.
- Protein aggregation: Recombinant antibodies can be prone to aggregation, reducing their efficacy and stability.
Read more: In vitro antibody production
No animals in recombinant antibody production
As mentioned above, one of the many benefits of recombinant antibody production is that there is no need for in vivo production steps using animals. While the immunization of a living animal like a mouse or rabbit is a part of hybridoma-based antibody production, the process of recombinant antibody production can be carried out without any animals.
Cell lines like Chinese Hamster Ovary cells (CHO cells), which are used for recombinant antibody production, are derived from Chinese hamster cells, but can be cultured in vitro. Hence, no hamsters are used in the process.
Not only is the use of in vitro cell cultures important for animal welfare, it also generates antibodies with a much higher specificity than those achieved through animal immunization methods.
Read more: In vitro antibody production
The role of evitria in recombinant antibody production
evitria AG offers recombinant antibody production services to customers worldwide. We focus on the production of recombinant antibodies using mammalian cell expression systems. Antibody production using CHO cells offers several advantages over bacterial and yeast systems. Mammalian cells are capable of performing complex post-translational modifications, such as glycosylation, that are necessary for the production of functional antibodies. Furthermore, our CHO cell expression platform allows the production of high volumes with no quality limitation.
Our expertise in producing recombinant antibodies makes us a valuable partner for companies and researchers in the biotechnology industry who require high-quality antibodies for various applications.
At evitria, our primary focus is on transient antibody expression in CHO. Nonetheless, we collaborate with a group of reliable partners who possess specialised expertise. No matter the nature of your project, evitria can provide you with the necessary support.
Find two of our workflows with trusted partners here:
Diagnostic Workflow
Therapeutic Workflow
Recombinant antibody production – conclusion
Recombinant antibody production is a powerful tool for generating high-quality antibodies for various applications, including research, diagnosis, and therapy. It involves the use of gene expression systems, such as bacterial or mammalian expression systems, to produce recombinant proteins that can then be purified and used as antibodies.
Recombinant DNA technology, combined with antibody engineering and optimization, has enabled the generation of antibodies with greatly improved efficiency and scalability, as well as improved antibody specificity, affinity, and compatibility with the human immune system, making them beneficial for therapeutic antibody production.
Recombinant antibody production is a rapidly evolving field with enormous potential for research, diagnosis, and therapy. By harnessing the power of genetic engineering and antibody optimization, it is possible to create highly specific and effective antibodies that can be used to address a wide range of biomedical challenges.
More readings about antibody production
- Expression of recombinant antibodies
- Purification of recombinant antibodies
- Recombinant protein production
- Monoclonal antibody production
- Polyclonal antibody production
- Antibody production
- 1.antibody. Encyclopedia Britannica. Accessed June 2024. https://www.britannica.com/science/antibody/Antibody-structure-and-classes
- 2.Lagassé HAD, Alexaki A, Simhadri VL, et al. Recent advances in (therapeutic protein) drug development. F1000Res. Published online February 7, 2017:113. doi:10.12688/f1000research.9970.1
- 3.Mark JKK, Lim CSY, Nordin F, Tye GJ. Expression of mammalian proteins for diagnostics and therapeutics: a review. Mol Biol Rep. Published online June 8, 2022:10593-10608. doi:10.1007/s11033-022-07651-3
- 4.Xu X, Zhang R, Chen X. Application of a single-chain fragment variable (scFv) antibody for the confirmatory diagnosis of hydatid disease in non-endemic areas. Electronic Journal of Biotechnology. 2017;29.
- 5.da Silva A, Corte-Real S, Goncalves J. Recombinant Antibodies as Therapeutic Agents. BioDrugs. 2008;22.
- 6.Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. Published online September 1, 2005:1126-1136. doi:10.1038/nbt1142
- 7.Huston JS, et al. . Medical applications of single-chain antibodies. Int Rev Immunol. 1993;10.
- 8.Hwang WYK, Foote J. Immunogenicity of engineered antibodies. Methods. Published online May 2005:3-10. doi:10.1016/j.ymeth.2005.01.001
- 9.Brinkmann U, Kontermann RE. The making of bispecific antibodies. mAbs. Published online January 10, 2017:182-212. doi:10.1080/19420862.2016.1268307
- 10.immunoconjugate. NCI Dictionary of Cancer Terms. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/immunoconjugate
- 11.Burdette LA, Leach SA, Wong HT, Tullman-Ercek D. Developing Gram-negative bacteria for the secretion of heterologous proteins. Microb Cell Fact. Published online December 2018. doi:10.1186/s12934-018-1041-5
- 12.Pereira S, Kildegaard HF, Andersen MR. Impact of CHO Metabolism on Cell Growth and Protein Production: An Overview of Toxic and Inhibiting Metabolites and Nutrients. Biotechnology Journal. Published online February 19, 2018. doi:10.1002/biot.201700499
- 13.Schütz A, Bernhard F, Berrow N, et al. A concise guide to choosing suitable gene expression systems for recombinant protein production. STAR Protocols. Published online December 2023:102572. doi:10.1016/j.xpro.2023.102572
- 14.Karbalaei M, Rezaee SA, Farsiani H. Pichia pastoris: A highly successful expression system for optimal synthesis of heterologous proteins. Journal Cellular Physiology. Published online February 14, 2020:5867-5881. doi:10.1002/jcp.29583
- 15.Stolfa G, Smonskey MT, Boniface R, et al. CHO‐Omics Review: The Impact of Current and Emerging Technologies on Chinese Hamster Ovary Based Bioproduction. Biotechnology Journal. Published online November 15, 2017. doi:10.1002/biot.201700227
- 16.Kim YJ, Han SK, Yoon S, Kim CW. Rich production media as a platform for CHO cell line development. AMB Express. 2020;10.