The use of recombinant antibody technology in the manufacture of therapeutics has rapidly expanded in the last two decades. Industry statistics indicate that there has been a more than $100 billion increase in the number of antibody drugs1, with a significant proportion of these being recombinant proteins produced in mammalian expression platforms2.
In this blog, we will review examples of recombinant proteins, the benefits and drawbacks of the technology in different expression systems, and the strategies in place to optimize manufacturing for research and industrial production for various applications in life sciences – from therapy and basic research to diagnostics.
Recombinant protein expression
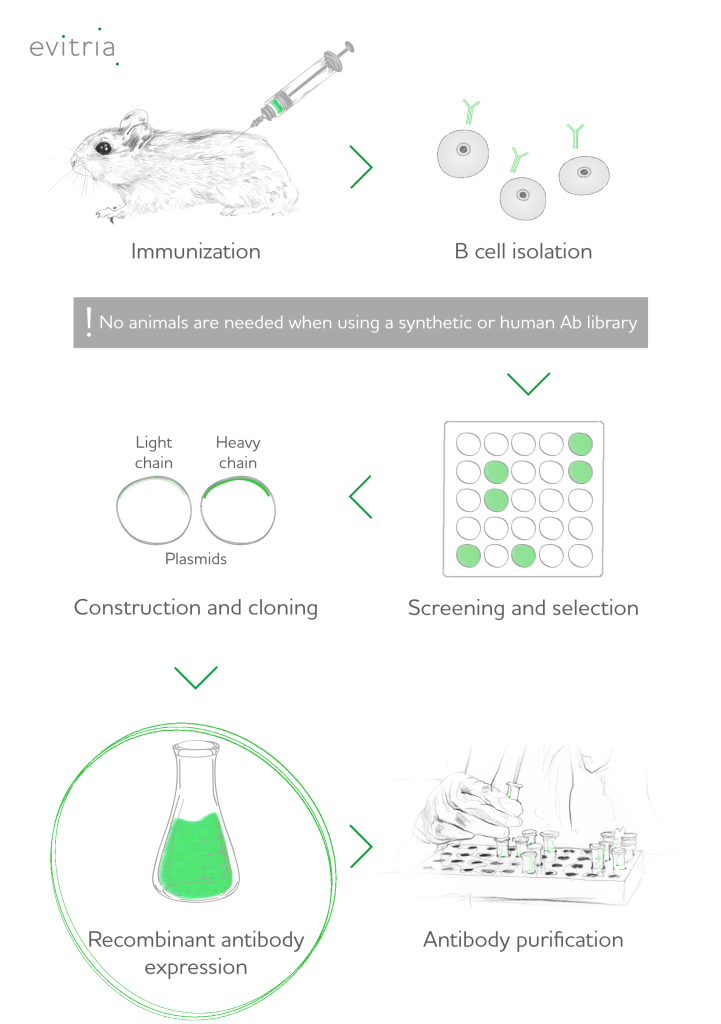
Recombinant proteins are produced via recombinant DNA technology: They are encoded by recombinant DNA clones into an expression vector. Subsequent transfer of the vector and the gene of interest in a suitable host results in gene expression, transcription and translation of mRNA (messenger RNA) to proteins.
The field of recombinant protein production is continuously evolving thanks to the culmination of research dating back to the early discoveries in DNA and protein synthesis to genetic engineering optimizations and breakthroughs in molecular biology. Today, recombinant technology is responsible for most of the protein therapeutics on the market, which are developed for cancer, immune disorders and other diseases.
Examples of recombinant proteins
Examples of recombinant proteins are
- Human insulin: treatment against diabetes
- Human growth factors: treatment for growth hormone deficiency
- Factor VIII: treatment for haemophilia
- Recombinant hormones and cytokines (e.g. interferons and interleukins)
- Therapeutic monoclonal antibodies, e.g. as a treatment for cancer or viral infections such as SARS-COV-2
- Research and diagnostic reagents, e.g. ELISA and fluorescence assays, inhibitors, receptors for small molecules, protein folding, antigens
Other examples include enzymes and protein fragments for vaccine production, recombinant antibodies for therapeutic and diagnostic applications (including bsAbs as an increasingly promising subtype) and associated labelling with additional residues to simplify characterization and chromatographic purification methods (e.g. GST).
Benefits of recombinant proteins
Recombinant protein expression has benefits over the conventional, historical methods. Prior to the rise of molecular biology and high-throughput recombinant protein technology, proteins had to be purified from animal sources. Recombinant protein expression is defined at the sequence level, therefore enabling high batch-to-batch reproducibility and is associated with fast and simple workflows. Historical methods would have purified protein from different sources, which was not only laborious, time-consuming and required large amounts of animal organs but also prone to contamination from other products that hampered the purity of the final manufactured product.
One example is insulin. Prior to the approval of the first biosynthetic human insulin product in 1982, and in concert with advancements in chromatographic processing, companies were able to produce purer insulin. The caveat of the method was the tremendous quantities of animal pancreases needed, which originated from cows or pigs. Using these methods, glands from 23,500 animals were needed to produce sufficient insulin to treat 750 patients with diabetes for a period of one year.3 Another significant factor that affected insulin was the availability of animal glands. The unsustainability of these methods was exemplified in the 1970s when the availability of animal glands declined, and the cost of beef and pork fluctuated.
Major benefits of recombinant proteins are:
- High Purity: Recombinant proteins can be produced with a high degree of purity, reducing the risk of contamination or impurities in experimental applications.
- Customization: Researchers can design and produce recombinant proteins with desired specificities, such as modifications, tags, or mutations to suit their experimental needs.
- Scalability: The possibility to scale up recombinant protein production makes it suitable for large-scale industrial and therapeutic applications.
- Consistency: The production of recombinant proteins can be tightly controlled, ensuring consistent quality and reproducibility in experiments, but also in drug manufacturing requiring large quantities.
- Cost-effectiveness: Compared to traditional methods that obtain proteins from natural sources, recombinant protein production can be more cost-effective, especially for rare or complex proteins.
Due to their manifold advantages, recombinant proteins are widely used tools all over the scientific landscape. For instance, researchers can design a recombinant protein as an antibody, which can bind to one specific target antigen in the body. This makes them unique weapons in the fight against several cancer types and other diseases.
Issues with recombinantly produced proteins
The production of recombinant proteins requires substantial resources and expertise. Failures in the manufacturing process can have severe consequences for patients and financial drawbacks.
Especially for therapeutic proteins, stringent quality control is indispensable to measure the bioactivity, stability and other main factors related to the quality and effectiveness of recombinant proteins.
Issues in recombinant protein production may include:
- Proteolysis
- Incorrect folding
- Formation of inclusion bodies
- Protein aggregation2
These drawbacks are often encountered in production with bacterial cells and can be circumvented by employing mammalian cells, such as HEK293 cells or CHO cells.
Recombinant protein production
Recombinant protein production techniques include:
- genetic engineering, DNA cloning and vector design (e.g. plasmid vectors)
- transferring DNA sequences of interest into host cells (transformation into bacterial, yeast, mammalian or transfection into mammalian cells)
- protein expression and purification (e.g. with SDS-page or affinity chromatography)
How are proteins changed to recombinant proteins?
Recombinant protein expression systems
Recombinant protein expression systems refer to some types of host cells that have proven superior for several protein synthesis needs:
- mammalian: for recombinant antibody production and highly complex proteins
- bacterial: for simple proteins, but is scalable at low cost to achieve high yields; simple culture conditions
- yeast: advanced protein processing, intermediate culture conditions
- insect cells: similar to mammalian protein processing, demanding culture conditions and time-consuming
Recombinant protein expression with mammalian systems
Recombinant protein expression with mammalian systems yields synthetic target proteins that are made up very much like their native counterparts. The reason is the very similar biochemistry production machinery, leading to similar post-translational modifications like glycosylation that closely match in vivo patterns.
Recombinant human proteins from mammalian systems, therefore, show very high physiological functionality and are preferred methods for functional assays and research. In vitro gene expression in simpler microorganisms such as Escherichia coli (E. coli) cannot provide the same high-quality proteins of interest.
Mammalian expression systems allow stable expression after the insertion of recombinant genes into the host cell line genome. More practically relevant is transient expression and protein purification of recombinant proteins in suspension mammalian cell culture (1-2 weeks). Optimized CHO cells yield titers of up to several grams per liter.
- 1.Zhang JH, Shan LL, Liang F, Du CY, Li JJ. Strategies and Considerations for Improving Recombinant Antibody Production and Quality in Chinese Hamster Ovary Cells. Front Bioeng Biotechnol. Published online March 4, 2022. doi:10.3389/fbioe.2022.856049
- 2.Tihanyi B, Nyitray L. Recent advances in CHO cell line development for recombinant protein production. Drug Discovery Today: Technologies. Published online December 2020:25-34. doi:10.1016/j.ddtec.2021.02.003
- 3.100 Years of Insulin. U.S. Food and Drug Administration. Published 2020. https://www.fda.gov/about-fda/fda-history-exhibits/100-years-insulin#:~:text=On%20October%2028%2C%201982%2C%20after,that%20derived%20from%20this%20technology