Afucosylated Antibody Expression Service
We provide afucosylated antibody expression services based on CHO cells. This enables us to express high-quality afucosylated antibodies for projects at different stages and scales – with no amino acid alterations.
Our expertise ensures that you receive afucosylated antibodies with enhanced Antibody-Dependent Cellular Cytotoxicity (ADCC), crucial for advancing therapeutic antibodies in immunotherapy and many other applications. We can achieve this by accessing ProBioGen’s proprietary GlymaxX® system, ensuring the absence of fucose residues without the need for altering the amino acid sequence of your antibody.
> 100
completed GlymaxX® projects
< 1 EU/mg
low endotoxin levels
> 175
GlymaxX® antibodies purified for customers worldwide
4 weeks
from antibody sequence to delivery
Rapid Afucosylated antibody production service to support your
development
There are many ways to enhance ADCC by Fc engineering (Table 1), but these require changing the amino acid sequence of your antibody – a big risk in any therapeutic development project.
Afucosylation, i.e. the removal of core fucose residues from antibody glycan structures is a way of enhancing ADCC without any amino acid substitutions. At evitria, we combine ProBioGen’s proprietary cell engineering approach, GlymaxX® with our transient CHO antibody expression platform to rapidly deliver high quality afucosylated antibodies.
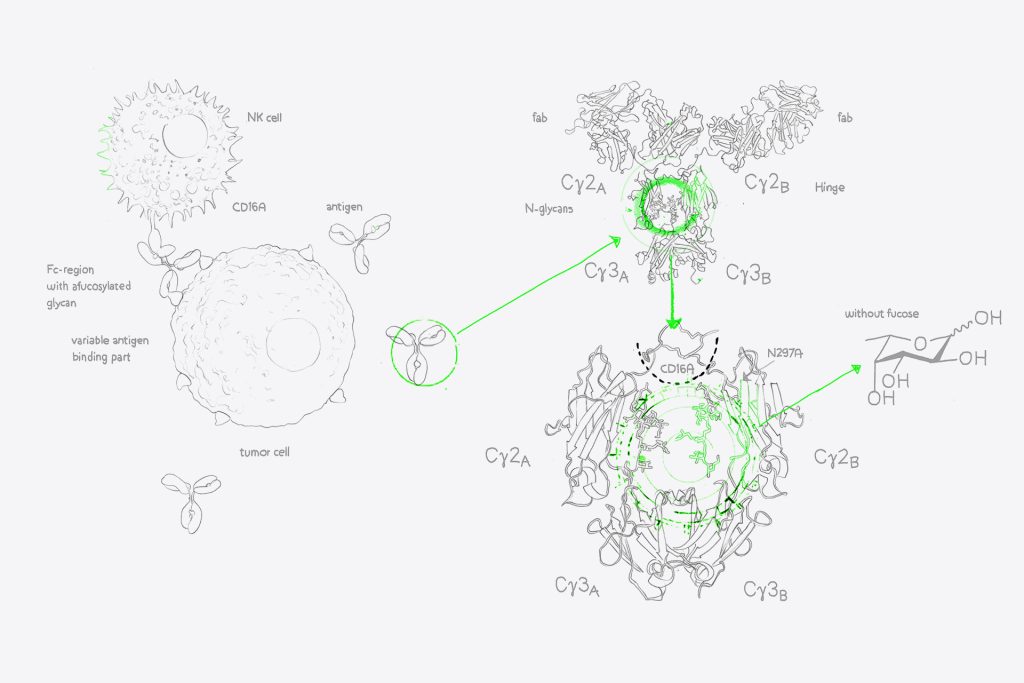
By increasing ADCC through afucosylation, your antibody drugs can achieve greater efficacy against target cells. This is achieved by enhancing the interaction between the antibody’s Fc region and FcγrIIIa receptors on effector cells like natural killer cells (NK cells) or T-cells, leading to improved antibody-dependent cell-mediated cytotoxicity.
Expression service for truly afucosylated antibodies? No matter of course!
ProBioGen’s GlymaxX® technology seamless integrates with our rapid transient CHO expression service. With these technologies, we can produce your ideal side-by-side comparison to study the impact of antibody afucosylation.
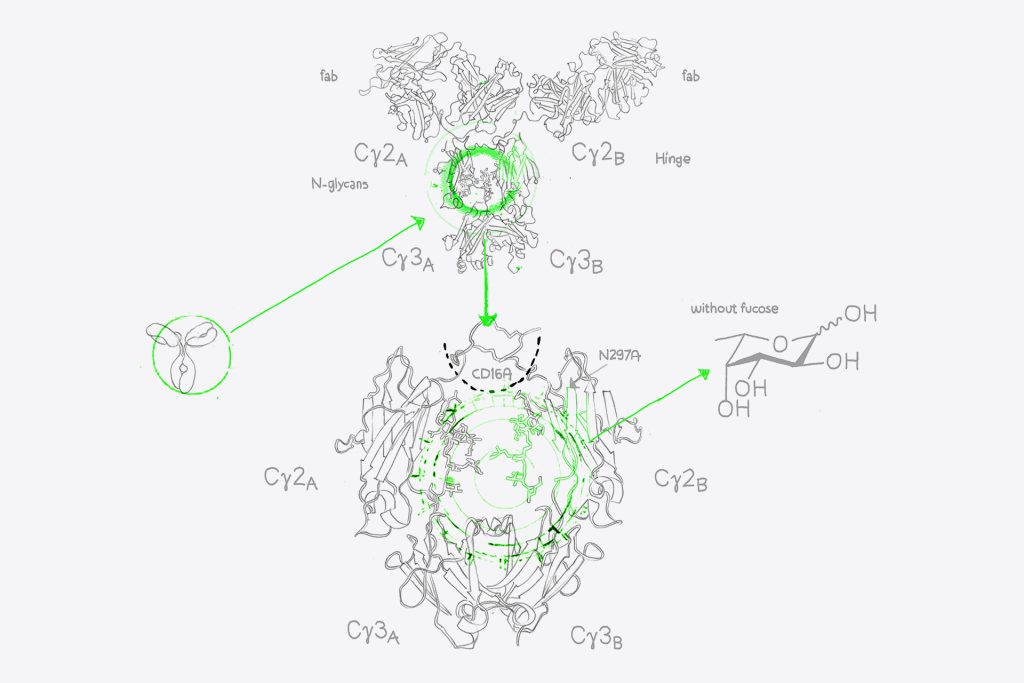
Simulataneously, we transfect one CHO cell culture with your recombinant monoclonal antibody, and another culture with your antibody plus the RDB enzyme of GlymaxX®. This enzyme redirects the fucose synthesis pathways within our CHO cells, and so prevents the addition of core fucose onto the Asn298 glycan structures within the antibody Fc domain. Both cultures grow, express the transfected antibody, and are purified in parallel using evitria’s robust plaform, delivering high quality material and the ideal wild-type control.
GlymaxX® avoids the risks associated with Fc mutations that could have an impact on immunogenicity, developability, and stability. By avoiding changes to the antibody’s oligosaccharide structures or N-linked glycosylation sites, we maintain the natural effector functions and stability of the antibody. We have verified this through mass spectrometry analysis, the gold standard of antibody characterisation, and can offer this enhanced analytics verification to any recombinant antibody production we perform.
We focus exclusively on recombinant antibody production through transient expression in CHO cells. This means that all our strength and expertise go into the production of mAbs, bsAbs, and other antibody-related formats – including afucosylated antibodies, Fc-silenced antibodies, and other proteins. Our dedicated team ensures rapid turnaround times and high-quality deliverables, so you can progress your research without delay.
Unsure on what afucosylated antibody you should use?
We’ve got you covered! Regardless of where your research is taking you, Dr. Desmond Schofield is dedicated to supporting you.

Dr. Desmond Schofield
Chief Business Officer at evitria
evitria’s afucosylated antibody expression service: accessing ProBioGen’s GlymaxX® technology
Our partnership with ProBioGen allows us to access the GlymaxX® platform – a cutting-edge technology for afucosylation. This system inhibits the enzymatic addition of fucose to the N-glycan structures on the Fc region of antibodies without any DNA sequence modifications. In other words: While alternative ADCC-enhancement approaches rely on Fc mutations that introduce risk of immunogenicity, developability, and stability risks, we can provide ADCC-enhanced materail without changing the sequence of your antibody.
By utilizing CHO host cells for our mammalian expression platform, we ensure high yields and scalability suitable for both small-scale assays and larger therapeutic antibody production. Our transient expression approach eliminates the need for stable cell line development, saving you time and resources.
With GlymaxX®, you receive antibodies with improved effector functions without compromising on quality or yield.
From sequence to antibody in 4 weeks
We understand how critical timelines are in biotechnology projects. Our efficient workflow allows us to provide you with afucosylated recombinant antibodies within just four weeks. We provide end-to-end services, from creating endotoxin-free plasmid DNA to high-throughput production of afucosylated antibodies using our CHO cell platform. Our process ensures high purity, detailed analytics—including glycosylation profiling and ADCC assays—and delivery of assay-ready antibodies for therapeutic development.
Frequently Asked Questions
How does evitria express afucosylated antibodies?
+What are the benefits of ProBioGen’s GlymaxX® platform?
+– Enhanced ADCC activity
– No sequence modifications
– Clinically validated