Fc-Silenced Antibody Expression Service
At evitria, we can express truly Fc-silenced antibodies at highest quality, thanks to STR technology and our CHO cell expression platform.
We understand the critical importance of eliminating unwanted immune system activation in several research and therapeutic applications.
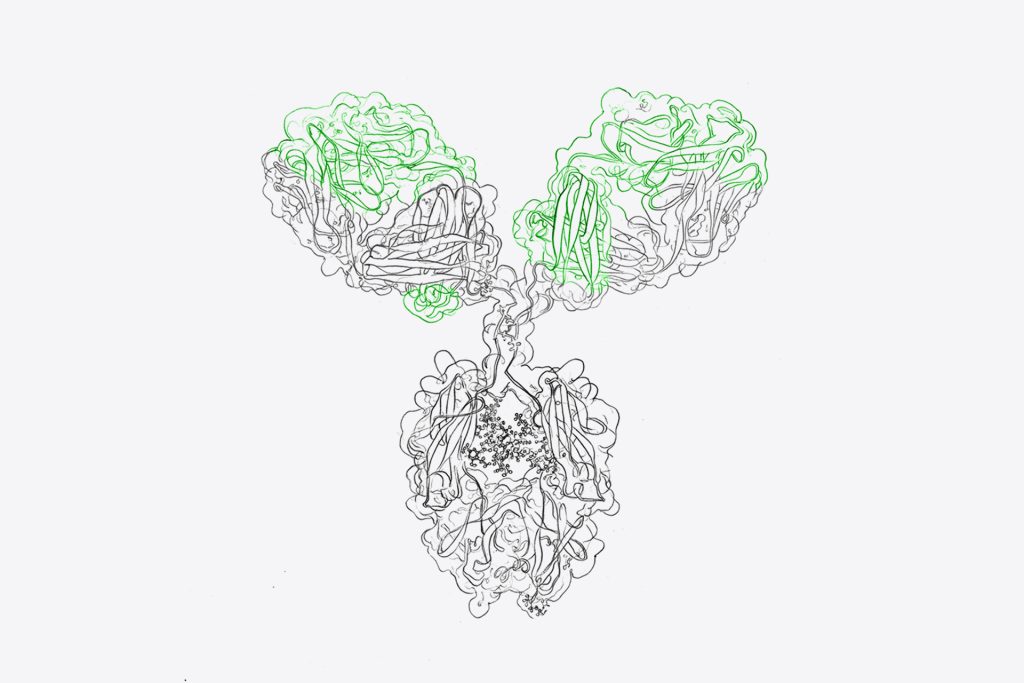
Through our partnership with mAbsolve, we utilize the innovative STR technology – a unique combination of three mutations in the Fc domain – that completely silences Fc gamma receptor interactions. This results in antibodies with zero activity above background noise, surpassing most of the previous efforts in Fc engineering.
0%
Fc activity
99%
projects successfully completed on time
20.000 +
antibodies purified overall for customers worldwide
4 weeks
from antibody sequence to delivery
Reliably Fc-silenced – mandatory for patient safety
For anti-viral antibodies, anti-inflammatory antibodies, and other therapeutic approaches using neutralizing antibodies, using Fc-silenced antibodies is essential to avoid unintended immune system activation that can interfere with therapeutic efficacy.
Therefore, our Fc-silenced antibodies completely and reliably eliminate immune effector functions mediated by the Fc region, such as binding to Fc gamma receptors on immune cells. This prevents side effects like cytokine release and antibody-dependent cell-mediated cytotoxicity (ADCC), maintaing precise antigen binding without triggering immune responses.
What makes evitria’s STR-modified Fc-silenced antibody production service stand out
evitria’s STR-modified Fc-silenced antibody production service offers unparalleled efficacy in completely eliminating Fc effector functions. Here’s what you can expect from us:
1. True Fc silencing with STR technology – Our antibodies incorporate mAbsolve’s STR mutations in the CH2 domain of the Fc region, achieving true Fc silencing with no residual activity. This is critical for antibody therapeutics where even minimal immune activity may pose a danger to patients.
2. Reduced side effects – By preventing binding to Fcγ receptors on immune cells like macrophages, B-cells, T-cells, and natural killer cells, our antibodies avoid triggering ADCC and cytokine release. This reduces potential side effects in therapeutic applications.
3. Recombinant production in CHO cells – We utilize recombinant antibody production in Chinese Hamster Ovary (CHO) cell lines, known for their reliability and high-quality protein expression. This ensures consistent glycosylation patterns and proper folding, essential for antibody stability and function.
4. Fast turnaround time – With our efficient workflow, we deliver antibodies from sequence to purified product in just 4 weeks. This rapid production cycle accelerates your research and development timelines.
5. Expertise in antibody engineering – Our team has extensive experience in antibody engineering, including Fc region modifications, to tailor antibodies to your specific needs. Whether you require human IgGs like human IgG1, murine IgGs like mouse IgG2a, or other species and isotypes, we can accommodate your requirements.
6. Comprehensive support – We offer technical support throughout your project, including assistance with experimental design, receptor binding assays, and interpretation of results.
7. Freedom to operate – Our partnership with mAbsolve ensures that you have the necessary licences for preclinical use, providing a clear commercialization pathway without intellectual property constraints.
By choosing evitria, you benefit from cutting-edge Fc silencing technology, reliable production methods, and a team dedicated to supporting your success in the lab and beyond.
Unsure on what Fc-silenced antibody you should use?
We’ve got you covered! Regardless of where your research is taking you, Dr. Desmond Schofield is dedicated to supporting you.

Dr. Desmond Schofield
Chief Business Officer at evitria
Expression service for truly Fc-silenced antibodies – and beyond
Beyond Fc-silenced antibodies, evitria offers a range of recombinant antibody production services. These include:
- Recombinant monoclonal antibody production service
- Afucosylated antibody production service
- Bispecific antibody production service
- Expression services for other proteins
Our expertise in pharmacokinetics, immunogenicity optimization, and Fc engineering allows us to customize antibodies for various applications. With our CHO cell expression platform, we ensure high-quality, scalable production to meet your project’s demands.
From sequence to antibody in 4 weeks
We know how tight timelines in biotechnological projects can be. With resources and know-how at hand, our efficient workflow allows us to provide you with Fc-silenced antibodies within only 4 weeks. Starting with molecular biology, large-scale expression commences after 2 weeks, followed by purification, extensive analytics, and – finally – shipment to our customers worldwide.